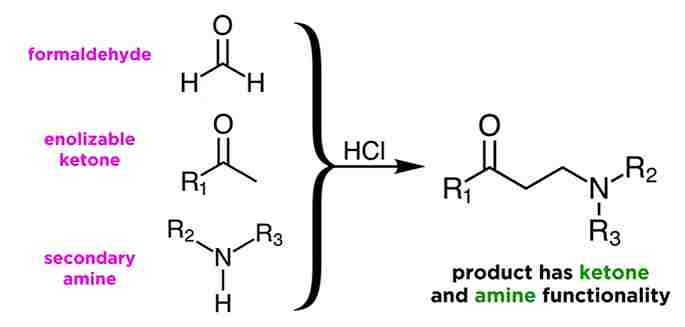
Mannich Reaction : Definition, Mechanism, Application, Explanation
Mannich Reaction PDF | Mannich Reaction Examples | Mannich Reaction Definition | Mannich Reaction Mechanism PDF | Mannich Reaction PPT | Mannich Reaction Procedure The Mannich Reaction was invented in 1912 by German chemist Carl Mannich, and has been the subject of study, extensions, and applications ever since. Definition: The condensation of a CH-activated compound … Read more