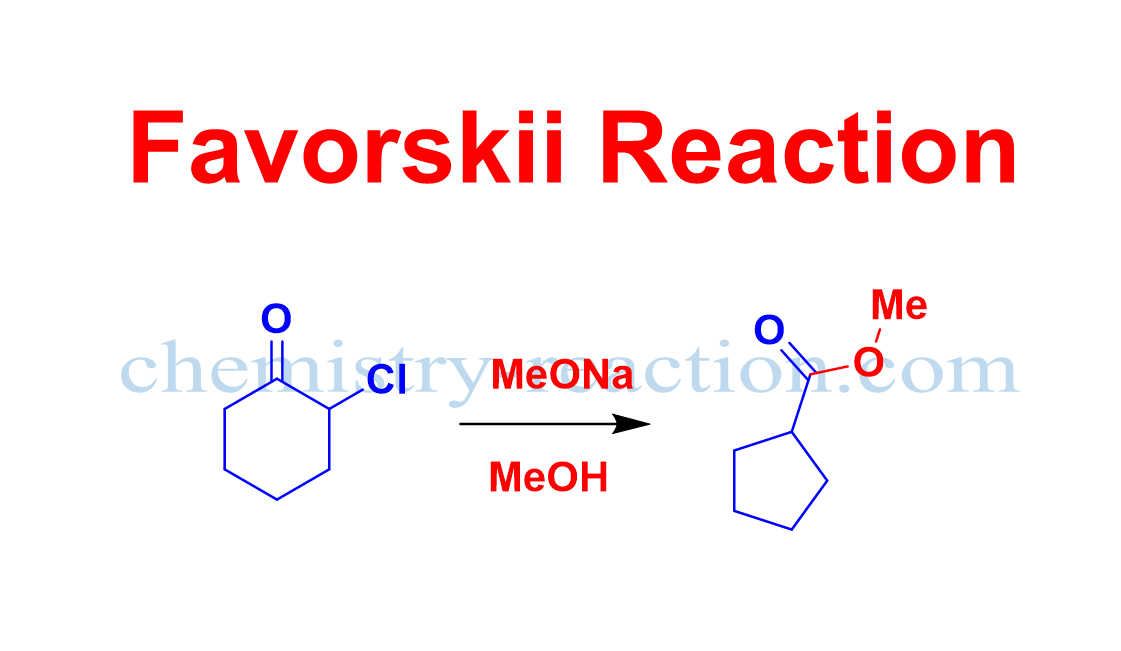
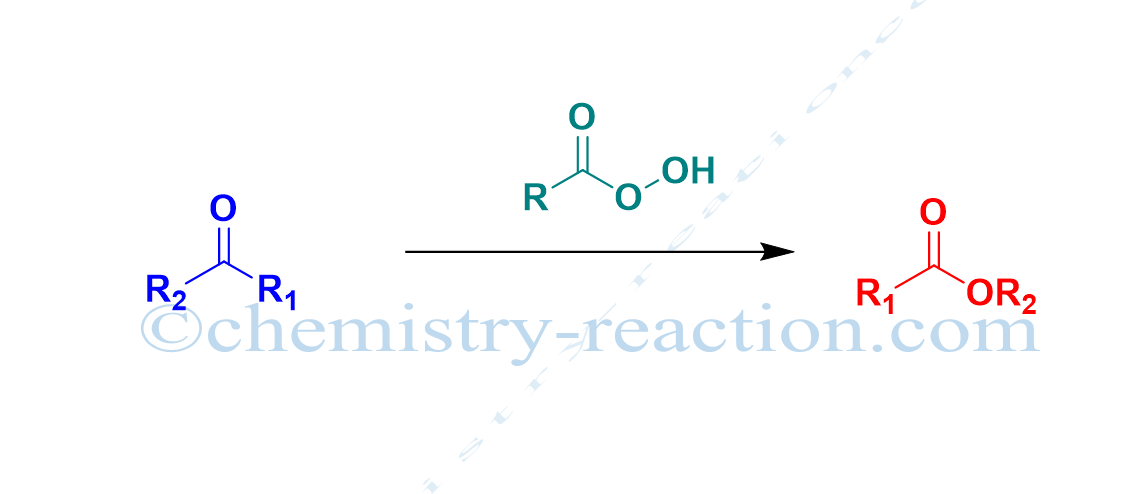
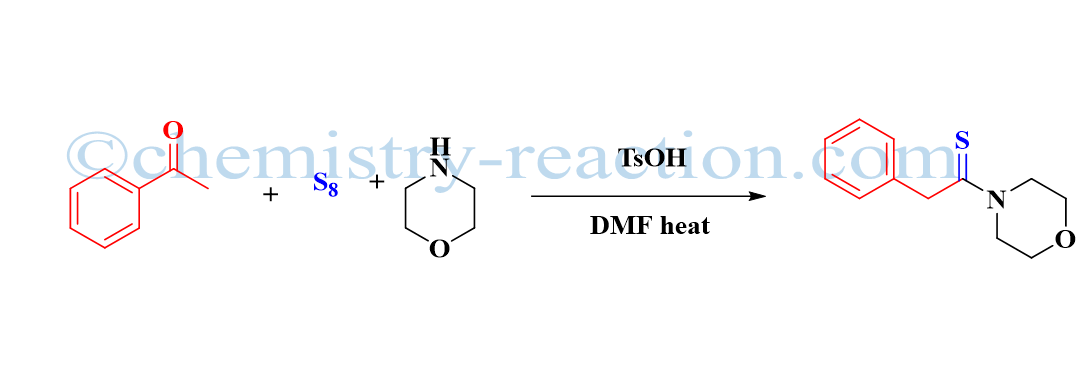
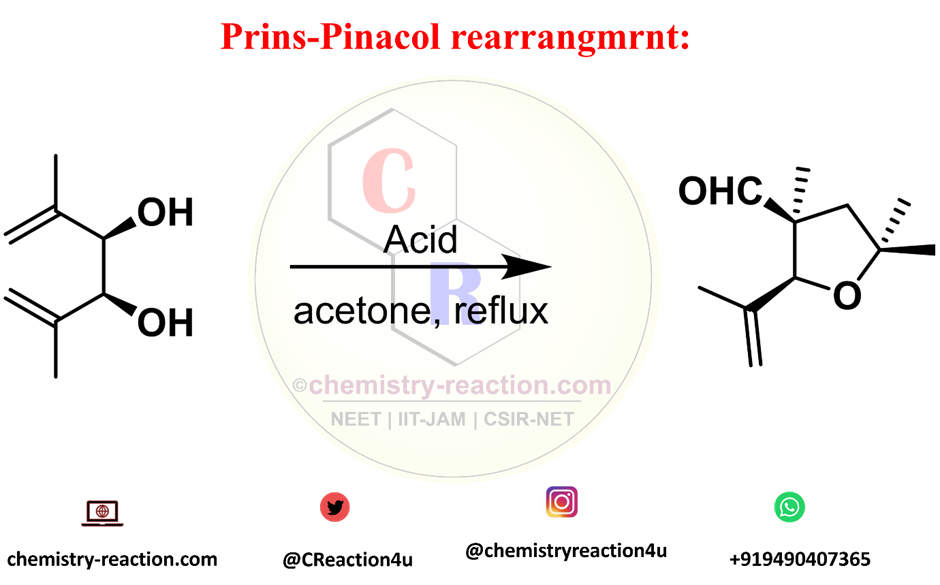
Aza-Claisen Rearrangement:
The Aza-Claisen rearrangement is a chemical reaction involving a nitrogen atom’s migration from one position to another within a molecule. It is named after the Claisen rearrangement, a well-known rearrangement reaction involving oxygen atoms. The mechanism of the aza-Claisen rearrangement: The mechanism of the aza-Claisen rearrangement involves three steps: Protonation: The reaction often starts with … Read more