Suzuki cross-coupling reaction is also known as Suzuki-Miyaura cross-coupling.
Table of Page Contents
what is Suzuki Coupling explain?
Suzuki cross -coupling reaction is an coupling reaction and also known as Suzuki-Miyaura cross-coupling, its coupling between a boronic acid and an organohalide or aromatic halides by using Palladium (0) complex is used to catalyst and base.
The Suzuki Couplings general used for formation of a carbon-carbon single bond between , organoboron substrate with the halides. This general scheme for the Suzuki coupling can be described as follows.
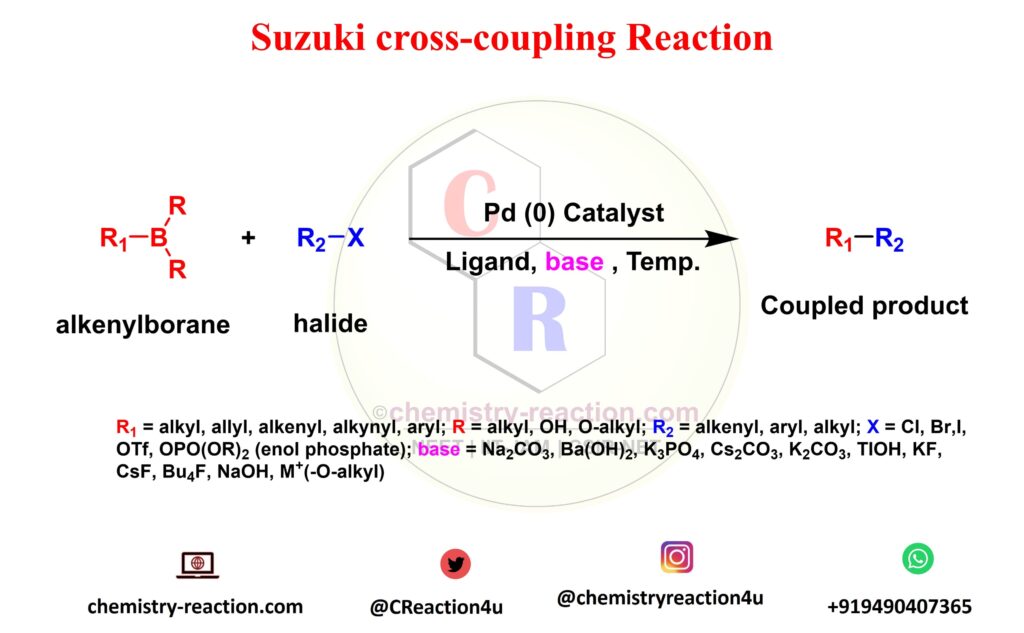
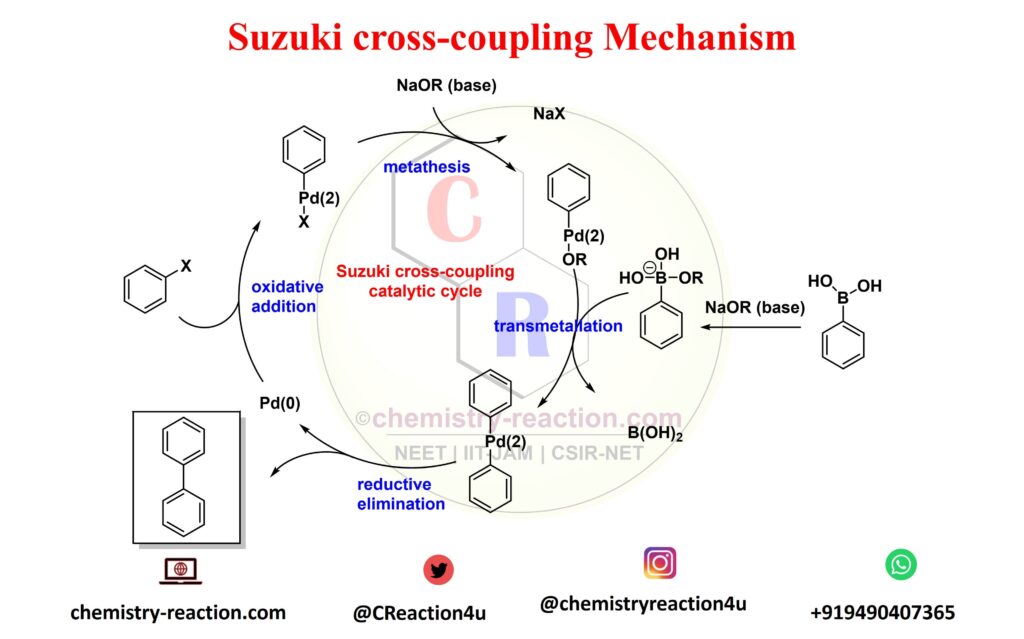
Suzuki Coupling Reaction Mechanism:
The Suzuki coupling mechanism follows a catalytic cycle involving three primary steps.
- Oxidative addition
- Trans-metalation
- Reductive elimination
The first step is the oxidative addition of aryl halides to the Pd(0) complex to give intermediate Ar-Pd-X, a Pd(2) species. Under the action of a NaOR (base), an organoborane compound reacts with intermediate Ar-Pd-X in transmetalation to give intermediate Ar-Pd(2)-Ar. This step is followed by reductive elimination to give the desired product and regenerate the original Pd(0) species.
Applications of Suzuki Reaction:
Suzuki reaction can join a sevral of alkenyl halides and aryl halides with arylboronic acids and alkenylboranes, so its became an essential strategy of synthesizing many biphenyls, styrenes, and alkenes. It is very affordable for use in the synthesis of intermediates for pharmaceuticals or fine chemicals.
Related Reaction:
- Chan-Lam coupling
- Heck reaction
- Hiyama coupling
- Kumada coupling
- Negishi coupling
- Petasis reaction
- Sonogashira coupling
- Stille reaction
References :
- Norio Miyaura, Kinji Yamada, Akira Suzuki, Tetrahedron Letters, 20,36,1979, 3437-3440,ISSN 0040-4039, https://doi.org/10.1016/S0040-4039(01)95429-2.
- Miyaura, Norio; Suzuki, Akira (1979), Chem. Comm. (19): 866–867. doi:10.1039/C39790000866
- Norio. Miyaura and Akira. Suzuki, Chem. Rev. 1995, 95, 7, 2457–2483
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
