Table of Page Contents
What is Favorskii Rearrangement explain?
Favorskii Rearrangement is organic reaction of α-halo ketones (chlorine, bromine, or iodine) having at least one α-hydrogen, with a nucleophile (alcohol, amine, or H2O) in the presence base (usually an alkoxide or hydroxide) give carboxylic acids or carboxylic acid derivatives via a cyclopropanone intermediate.
It is widely used organic reaction for the synthesis of very branched carbonyl compound like carboxylic acids, ester, and amide)
Ring-contraction reaction is happening when cyclic α-halo ketone substrates is used for reaction
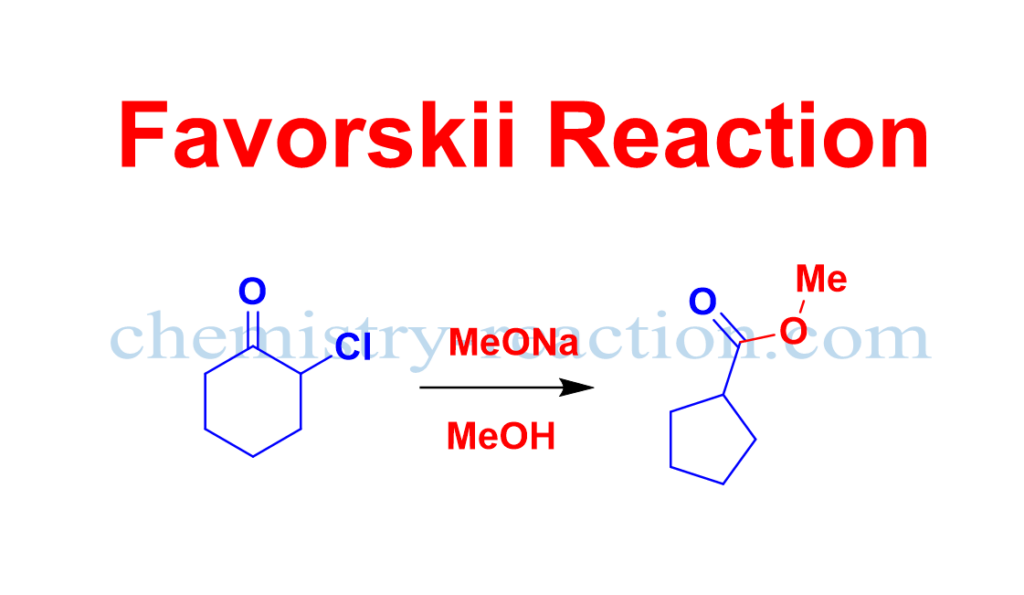
Favorskii Rearrangement Mechanism:
In the first step enolate will form by deprotonation at the α-carbon of α-halo ketones. In the second step enolate will attack (intramolecular attack) on the α’-carbon bearing the leaving group (chlorine, bromine, or iodine) to form a cyclopropanone intermediate. Then the nucleophile will attack on the carbonyl carbon of cyclopropanone intermediate and ring will open respectively at the most stable carbanion.
At last, the protonation of the carbanion gives the product.
Currently the widely accepted proposed mechanism of the Favorskii rearrangement are shown below.

Related Reactions and modification:
- Homo-Favorskii rearrangement
- Quasi-Favorskii rearrangement
References :
- Cope, Arthur (1960). Organic Reaction Volume XI (1 ed.). New York: Wiley-Interscience. doi:10.1002/jps.2600500225. ISBN 9780471171270.
- Org. Synth. 1977, 56, 107
- J. Org. Chem. 43 (14): 2936–2938.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

2 thoughts on “Favorskii Rearrangement”