Table of Page Contents
What is Ritter reaction explain?
It is an organic reaction fused for the Synthesis of N-tert-alkylamides from nitriles and alkenes or tertiary alcohols under strongly acidic conditions is referred to as the Ritter reaction.
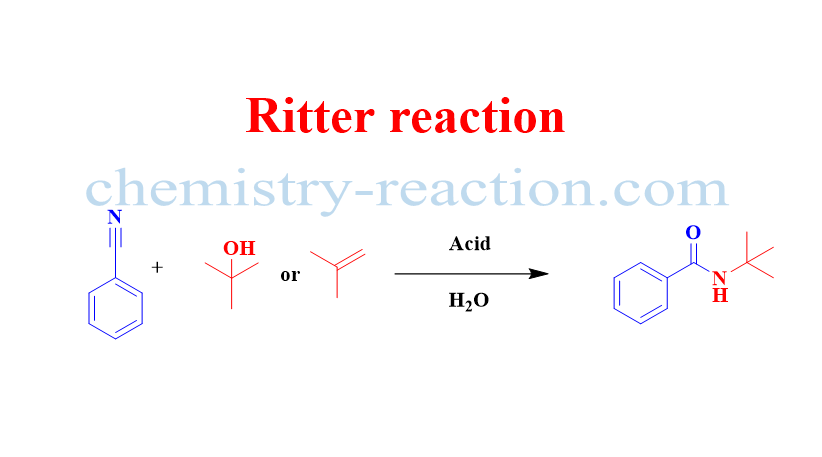
What is the purpose of the Ritter reaction?
The preparation of N-alkyl carboxamides from aliphatic or aromatic nitriles and carbocations is known as the Ritter reaction.
What kind of amides are prepared by ritter reaction?
Ritter reaction is widely used for the preparation of secondary acyclic amides as well as heterocycles (e.g., lactams, oxazolines, dihydroisoquinolines).
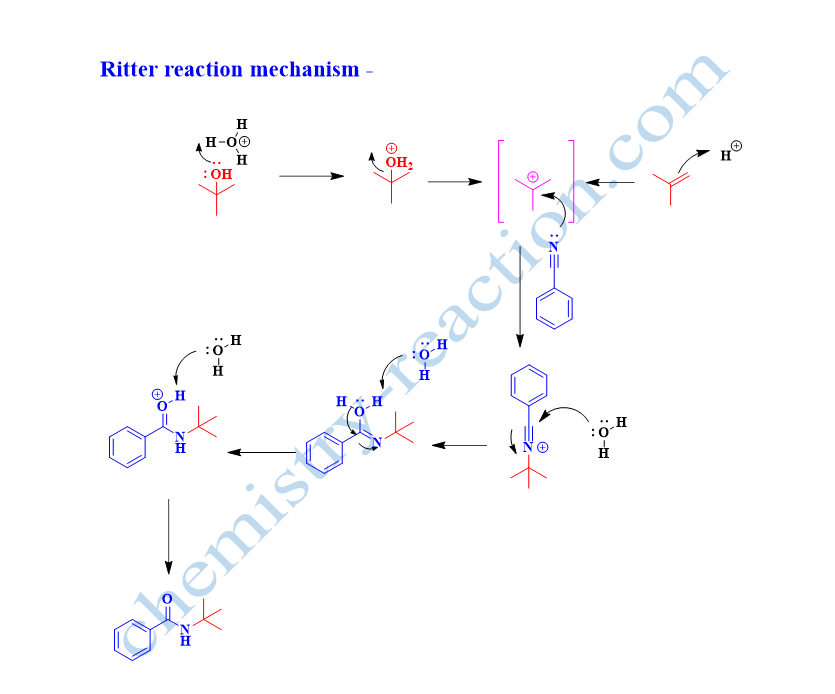
Ritter Reaction Mechanism –
In the mechanism of the Ritter reaction, whenalcohol is used as starting material, the strong acid will protonate the O-H group of alcohol and generate stable carbocation by heterolytic cleavage of the C-O bond.
The nitrogen atom of the nitrile will attack on carbocation intermediate and generate nitrilium ion which will react further with conjugate base of acid and give imidate intermediate, hydrolysis of imidate gives desired N-alkyl carboxamide produces.
Additional information-
- Prominently Ritter reaction is for synthesis of Heterocyclic compounds and secondary amides.
- Tertiary-, secondary, or benzylic alcohols, alkenes or alkyl halides are can be used for the generation of carbocation.
- Acid sensitive functional group of nitrile containing substrate will not sustain in strong acidic condition.
- Protic acids, Lewis acids (e.g., SnCl4, BF3·OEt2, AlCl3, etc.) have been successfully employed in the Ritter reaction for generate of carbocations
- 2° and 3° alcohols, benzylic alcohols are the best substrate for for carbocation generation bcz it will ionize easily.
Related Reactions:
References:
| 1. 2. | Ritter, J. J.; Minieri, P. P. J. Am. Chem. Soc. 1948, 70, 4045–4048. Ritter, J. J.; Kalish, J. J. Am. Chem. Soc. 1948, 70, 4048–4050. |
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “Ritter Reaction”