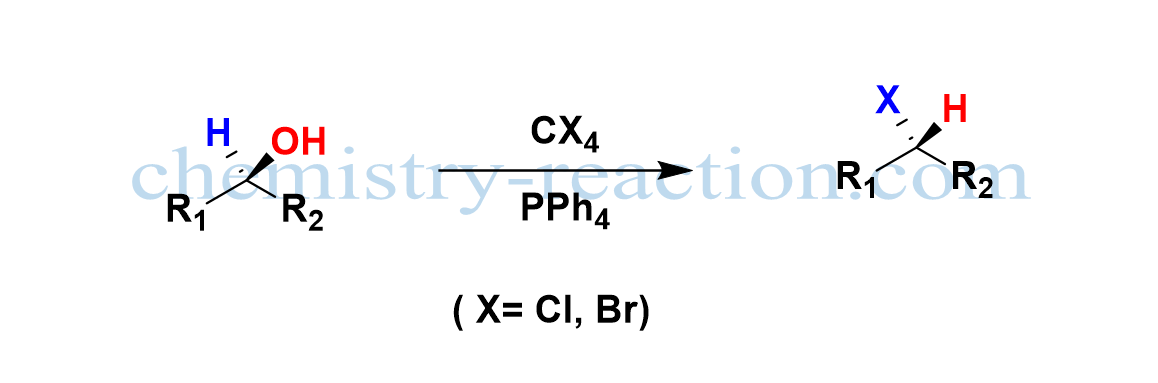Name Reaction
Simmons-Smith Reaction Mechanism
Simmon Smith reaction is most powerful organic cheletropic (reaction) method for cyclopropane preparation from diiodomethane (CH2I2) in the presence of zinc-copper couple (Zn-Cu) from unfunctionalized alkenes (e.g., cyclohexene, styrene) stereospecifically.
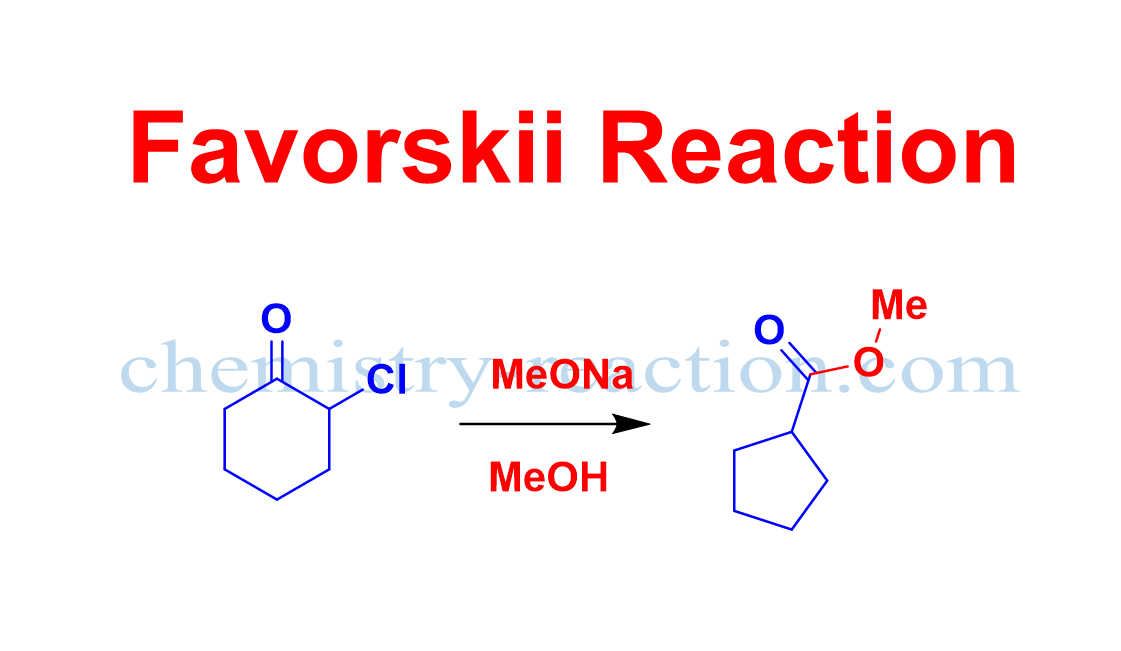
Favorskii Rearrangement
What is Favorskii Rearrangement explain? Favorskii Rearrangement is organic reaction of α-halo ketones (chlorine, bromine, or iodine) having at least one α-hydrogen, with a nucleophile (alcohol, amine, or H2O) in the presence base (usually an alkoxide or hydroxide) give carboxylic acids or carboxylic acid derivatives via a cyclopropanone intermediate. It is widely used organic reaction … Read more
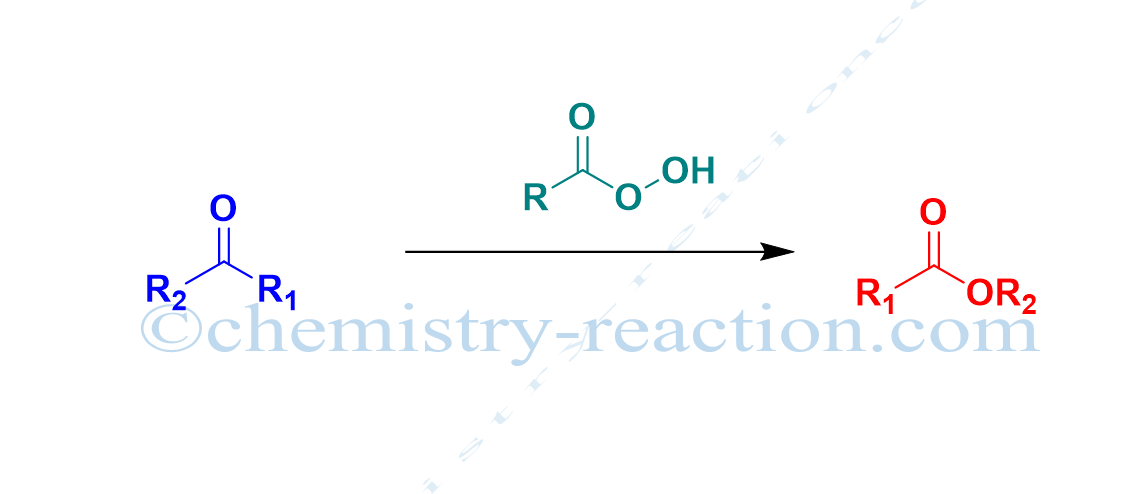
Baeyer-Villiger Rearrangement (Baeyer-Villiger Oxidation):
The conversion of ketone (cyclic ketone, aromatic ketone, aliphatic ketone) into corresponding esters (lactones) by using MCBPA, or hydrogen peroxide as oxidant and a Lewis acid is known as Baeyer-Villiger oxidation (Baeyer-Villeger Reaction).
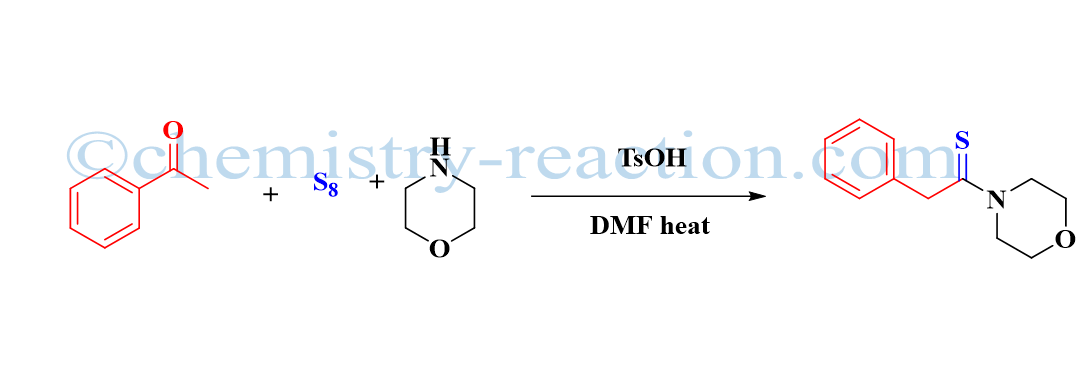
Willgerodt-Kindler Reaction
The willgerodt kindler reaction is the modification of the Willgerodt Reaction. It comes under a Rearrangement reaction. The name has been given after Karl Kindler.
Appel Reaction:
The Appel reaction is a common organic nucleophilic substitution reaction (SN2) employed to transform alcohol to a corresponding alkyl halide using the reagents tetrahalomethane (CX4) and triphenylphosphine (PPh4) with inversion of stereochemistry and high yield.