What is Takai-Utimoto Olefination?.
Takai reaction is a general carbon-carbon bond-forming reaction in organic chemistry. Takai reaction is also known as takai -Utimoto olefination; this transformation is referred to as the name Kazuhiko Takai.
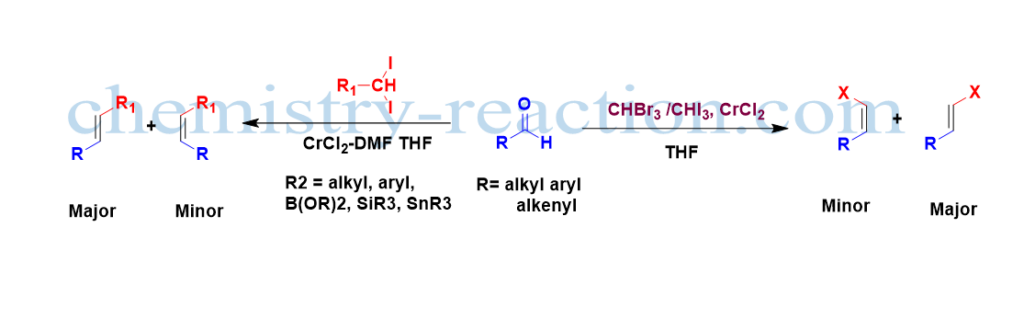
This olefination is a simple alternative for the Wittig reaction and other complex methods like preparing an E alkene stereoselective (major) isomer. In this method, chromium(II) catalyst was used to prepare (E) configuration of alkenyl halides from corresponding carbonyl compounds like aldehyde and ketones.
Compared to aromatic and aliphatic aldehydes, stereoselectivity is poor in the case of α and β-unsaturated aldehydes. The reaction rate is I>Br>Cl; because iodoform requires a low temperature for oxidative additions compared to other halogens. Aldehydes react faster than ketone.
TAKAI-UTIMOTO OLEFINATION (TAKAI REACTION) MECHANISM:
The following Takai Reaction mechanism is proposed reaction mechanism and refed from book. However, the Takai reaction proceeds stepwise, first oxidative addition of CrCl2 will take place in C-I bond of alkyl halide and generate geminal dichromium intermediates, Cr+2 toCr+3. This dichromium species is reactive and nucleophilic that attack the carbonyl compound (aldehyde or ketone) and form β-oxychromium species. The (E)-alkene is formed from the β-oxychromium species.

Related reaction :
References:
- Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms – J. Chem. Educ. 2005, 82, 12, 1780
This olefination is a simple alternative for the Wittig reaction and other complex methods like preparing an E alkene stereoselective (major) isomer. In this method, chromium(II) catalyst was used to prepare (E) configuration of alkenyl halides from corresponding carbonyl compounds like aldehyde and ketones.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.


1 thought on “TAKAI-UTIMOTO OLEFINATION (TAKAI REACTION)”