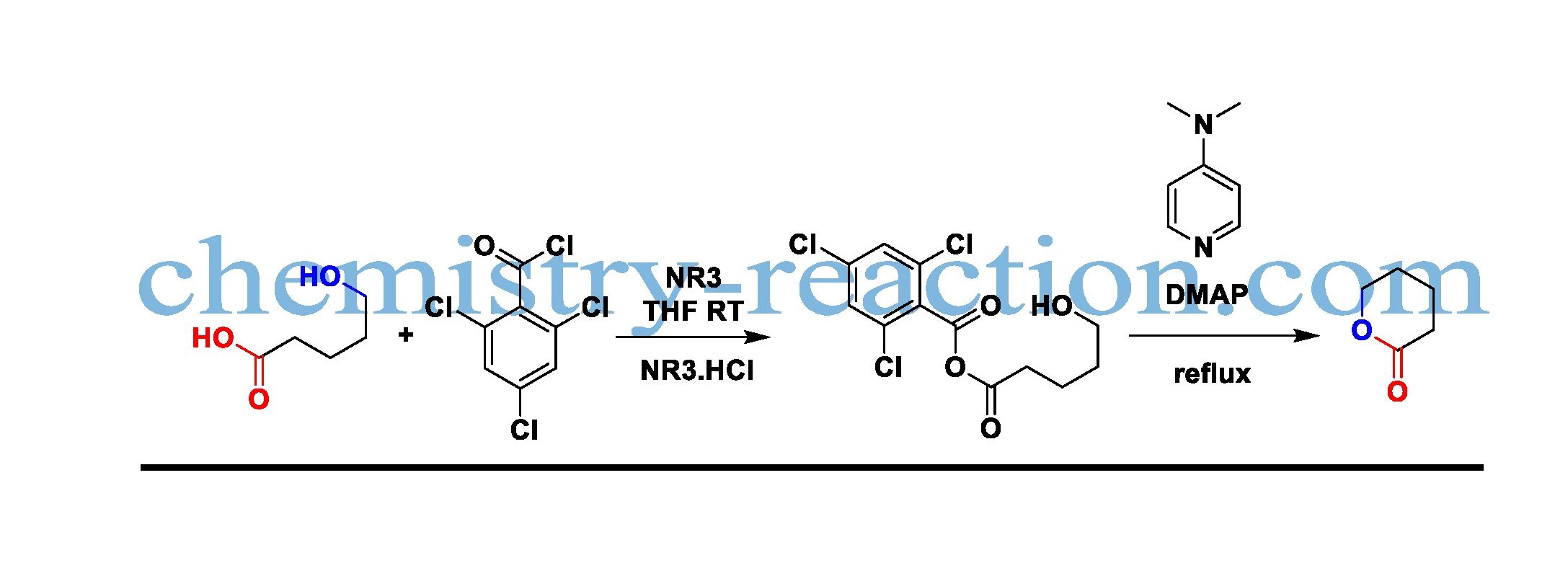
The development of high number ring lactones from hydroxy acids employing Yamaguchi reagent (2,4,6-trichlorobenzoyl chloride/DMAP) is known as the Yamaguchi macrolactonization
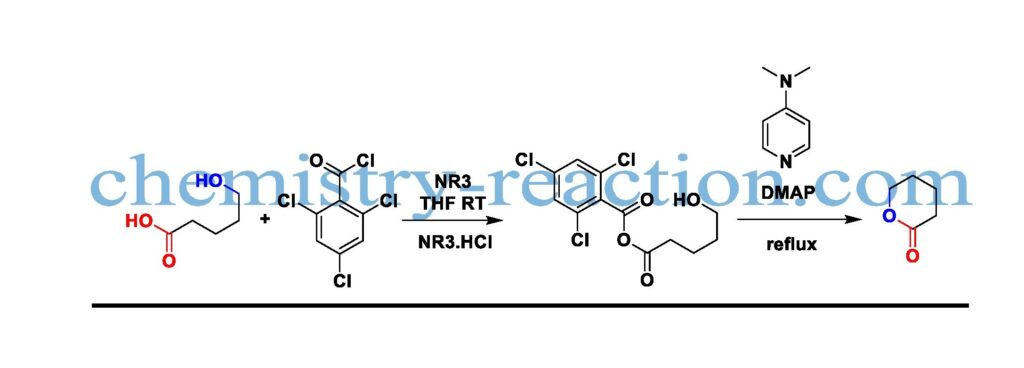
The best solvent forms this transformation is aliphatic hydrocarbon; Yamaguchi macrolactonization is used to prepare lactones and esters of the corresponding mixed anhydrides. The starting material acid was reacted with 2,4,6-trichlorobenzoyl chloride in the presence of NEt3, by removing triethylamine hydrochloride.
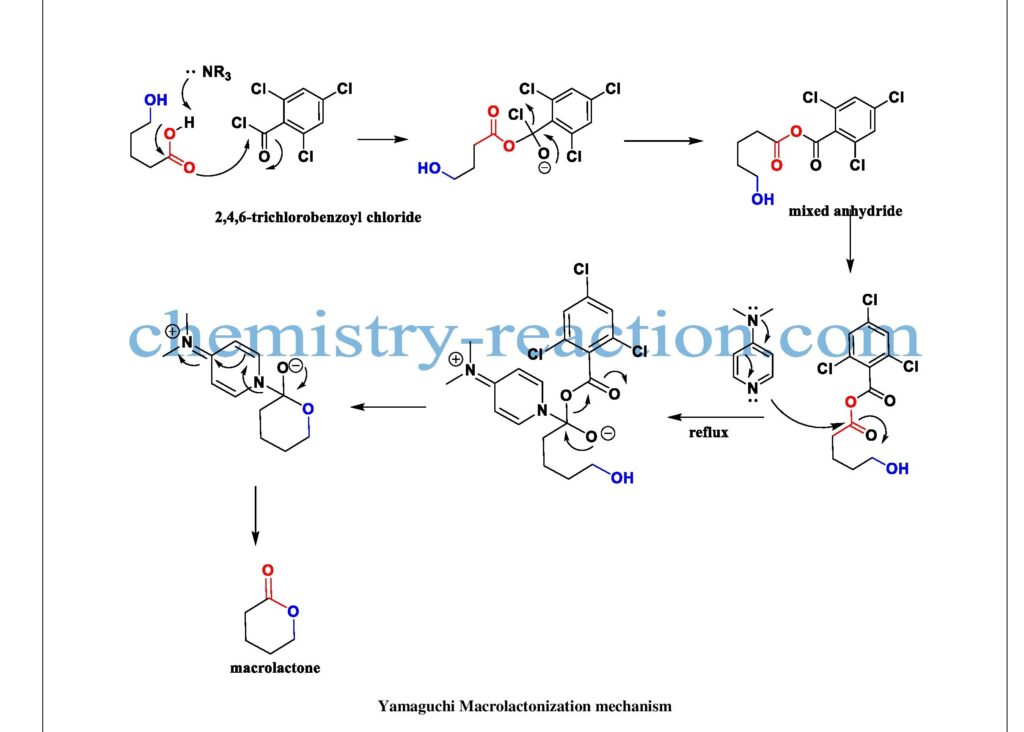
The formed mixed anhydride after refluxing with DMAP The desired product macrolactone will form. To avoid intramolecular coupling, product dilute is more with respective solvent. DMAP reagent mechanism and Yamaguchi mechanism are shown below
Yamaguchi Macrolactonization Mechanism
Do subscribe and ask in comment for Yamaguchi esterification ppt, Yamaguchi esterification pdf, and DMAP reagent mechanism.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.