Smith-Tietze Multicomponent Dithiane Linchpin Coupling (Smith-Tietze coupling) is the one-pot multicomponent coupling of 2-silylated-1,3-dithianes with epoxides.
The first application of 2-lithio-1,3-dithianes as “carbonyl anion” equivalents was described by E.J. Corey and D. Seebach in the mid-1960s.
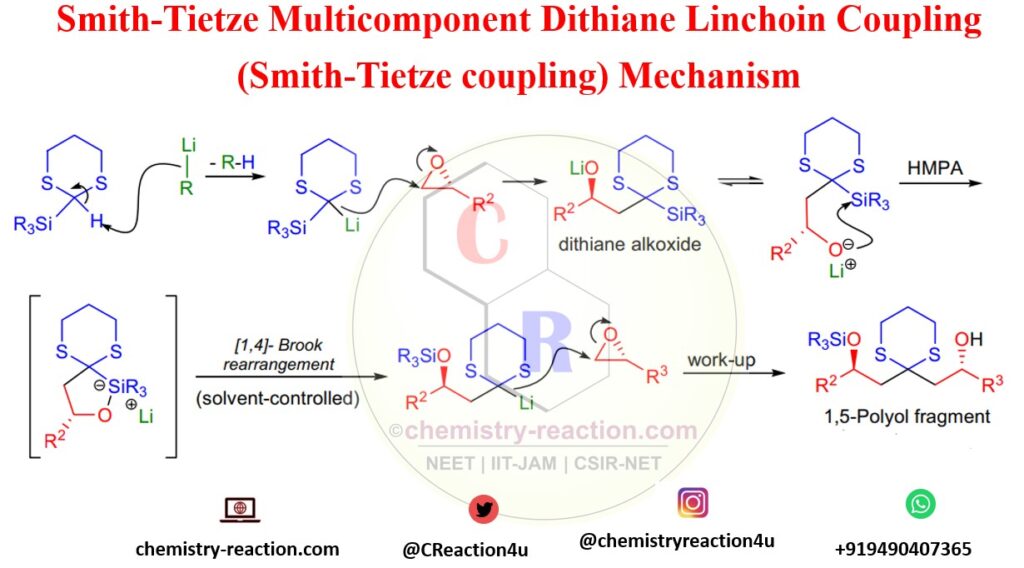
Smith-Tietze Multicomponent Dithiane Linchpin coupling protocol began with the deprotonation of the 2-trialkylsilyl-1,3-dithiane with an alkyl lithium followed by the addition of 2.2 equivalents of epoxide in the presence of one equivalent of crown ether. After the opening of the first epoxide, the resulting alkoxide intermediate underwent a spontaneous [1,4]-Brook rearrangement, thus generating a second dithiane anion that reacted with the remaining excess epoxide. Tietze–Smith Linchpin Reaction is a multicomponent coupling protocol, however, had a long reaction time, and it was unsuitable for unsymmetrical couplings. A.B. Smith et al. used HMPA or DMPU as an additive in the solvent, which significantly increased the rate of the reaction and allowed two different electrophiles (epoxides) to be coupled with the dithiane in a one-pot operation.

Table of Page Contents
The Smith-Tietze coupling advantages:
1) In the Tietze–Smith Linchpin Reaction Optically active terminal epoxides can be readily prepared by known methods.
2) The epoxide ring-opening is completely regioselective, the nucleophile attacks on the least substituted carbon.
3) The exact timing of the Brook rearrangement is possible by the addition of HMPA or DMPU to the reaction mixture (solvent-controlled Brook rearrangement) and the formation of symmetrical adducts can be completely avoided;
4) Altering the absolute configuration of the epoxides and the stereoselective reduction of the ketone moiety after the removal of the dithiane can give rise to 1,3-polyols of any desired configuration.
5) After the second epoxide has reacted, the resulting unsymmetrical adduct has its hydroxyl groups differentiated by one of them being silylated.
6) The use of an enantiopure bis-epoxide as the second epoxide component allows for a one-pot five-component linchpin coupling
Mechanism of the The Smith-Tietze coupling:
The key step of the mechanism is the solvent-controlled [1,4]-Brook-rearrangement, which proceeds through an intermediate having a pentacoordinate-silicon atom. This rearrangement does not take place until HMPA is added to the solvent. A similar solvent effect has been observed by K. Oshima, K. Utimoto and co-workers. The rearrangement was found to be completely intramolecular based on the results of a crossover experiment by A.B. Smith et.al.
Applications of Smith-Tietze coupling:
The Total Synthesis of the Anticancer Australian Rainforest Polyketide EBC-23
Related Reaction:
References :
- J. Am. Chem. Soc. 1997, 119, 29, 6925–6926
- J. Am. Chem. Soc. 2003, 125, 47, 14435–14445
- https://onlinelibrary.wiley.com/doi/10.1002/anie.201509342
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
