Staudinger reaction is an organic name reaction of organic azides with trivalent phosphorous compounds (e.g., trialkyl- or triarylphosphines) to afford the corresponding aza-ylides.
In 1919, H. Staudinger and J. Meyer reported the reaction between phenyl azide and triphenylphosphine, which afforded a novel compound, phosphinimine (also known as aza-ylide or iminophosphorane), in quantitative yield accompanied by the evolution of nitrogen gas.
It was found that benzoyl azide reacted with triphenylphosphine in an analogous fashion to afford the corresponding benzoyl aza-ylide. The authors also investigated the reactivity of phosphinimines and demonstrated that the reaction of carbon dioxide with phenyl aza-ylide gave rise to phenyl isocyanate and triphenylphosphine oxide, which is the first example of an aza-Wittig reaction.
Table of Page Contents
The general features of Staudinger Reaction:
Staudinger reaction experimental procedure and conditions:
in the staudinger reaction protocol Azide and diphenyldisiloxane (DPDS) were combined in a glass vial containing a magnetic stir bar. The mixture was dissolved in Staudinger reaction solvent that is CPME, and Ph3P was added. Gas evolves from the reaction immediately. The reaction was left to stir at room temperature for 24 h, after which it was quenched by the addition of water.
Staudinger reaction workup:
The Staudinger reaction mixture was allowed to stir until gas stopped evolving, and subsequently, the mixture was diluted two times with Et2O. The desired amine was obtained as the corresponding hydrochloride salt by precipitation with 0.5 mL of 4.0 M HCl in dioxane. it is very fast and deliver quantitative yield without the formation of side products.

Reduction | Staudinger Azide Reduction: synthesis of amine
Mechanism of the Staudinger reaction:
The mechanism of the Staudinger reaction has been subject to several studies and at this point the exact mechanism remains unclear. According to experimental data that free radicals or nitrenes are not intermediates in this transformation.
The first step of the mechanism is the attack of trivalent phosphorous by the unsubstituted nitrogen atom (N ) of the azide to give the subsequent phosphazide (which occasionally can be isolated) with retention of configuration at the phosphorous atom.
Next, the phosphazide goes through a four-membered transition state, which upon losing dinitrogen affords the iminophosphorane. A subtle point in the mechanism is the exact mode of attack of the phosphorous at N , since the PN N N backbone is not linear. Instead, the ANNN angle is approximately.
There are two possible trajectories of the phosphorous atom to approach N in Staudinger reaction:
1) from the same side of the R1 substituent on N trans attack.
2) and another is the opposite side of the R1 substituent on N cis attack

Applications of Staudinger Reduction:
The iminophosphoranes are versatile synthetic intermediates can be used :
1) hydrolysis with water gives primary amines (this reduction of azides is highly chemo- and stereoselective). Intermolecular or intramolecular reaction with carbonyl or thiocarbonyl compounds affords imines (Aza-Wittig reaction)
2) carboxylic acids convert iminophosphoranes to N-substituted amides. acyl halides condense to generate imydoyl halides; and ozonolysis produces nitro compounds.
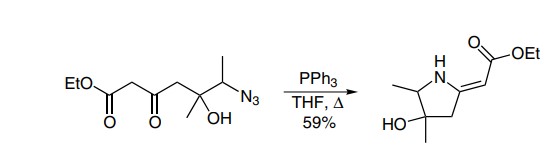
Staudinger reaction example:
Related Reaction/Staudinger type reaction:
- Staudinger ketene cycloaddition
- staudinger ligation reaction
- staudinger aza wittig reaction
- staudinger-vilarrasa reaction
- staudinger-phosphite reaction
- staudinger–aza-wittig reaction
References :
- J. Org. Chem. 2019, 84, 10, 6536–6545
- https://www.organic-chemistry.org/namedreactions/staudinger-reaction.shtm
- https://pubs.acs.org/doi/10.1021/acs.chemrev.9b00665
- https://www.sciencedirect.com/staudinger-azide-reduction
- https://onlinelibrary.wiley.com/doi/10.1002/9780470638859.conrr595
- staudinger reaction review
- staudinger-reaction General Characteristics.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
