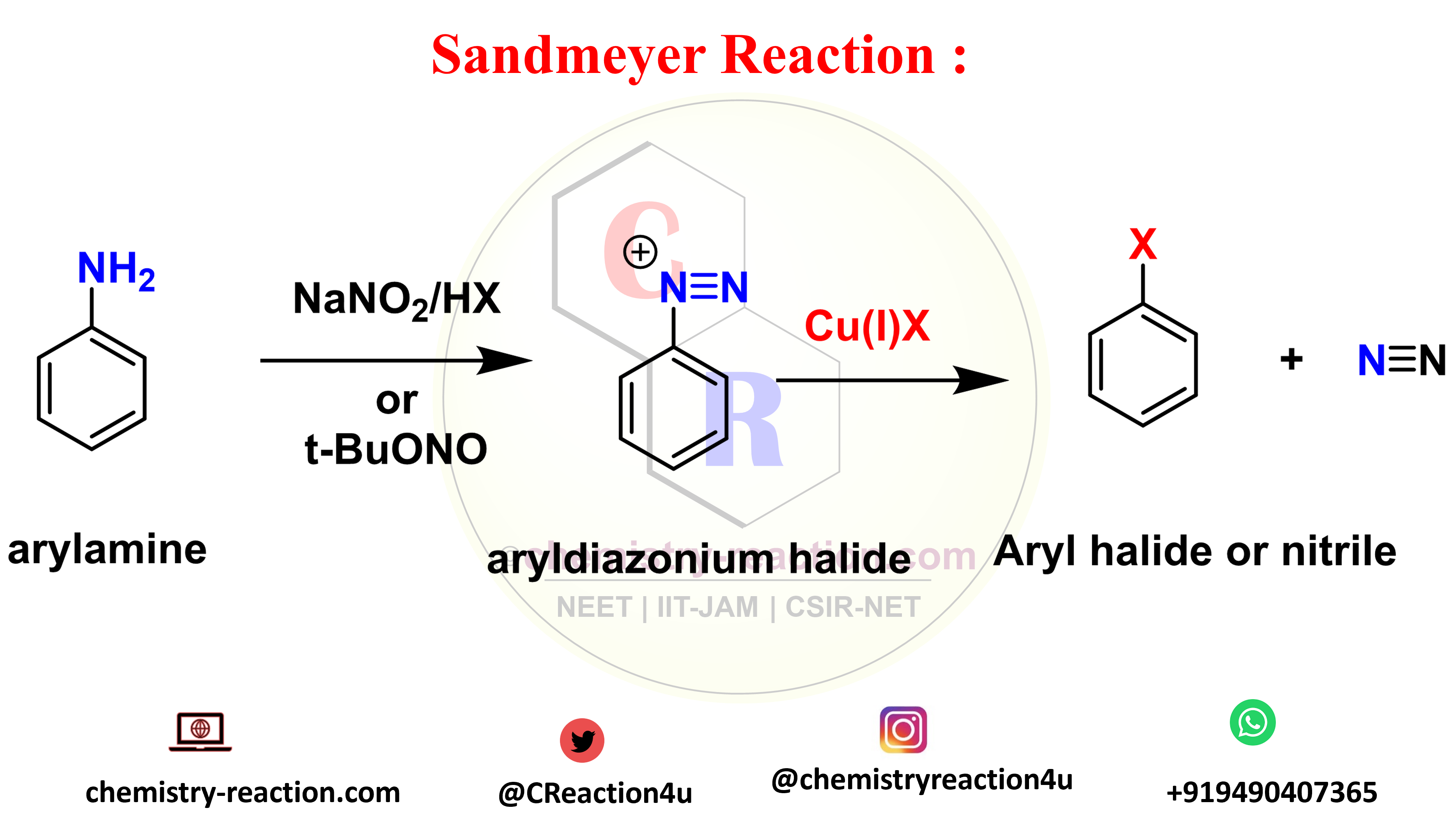
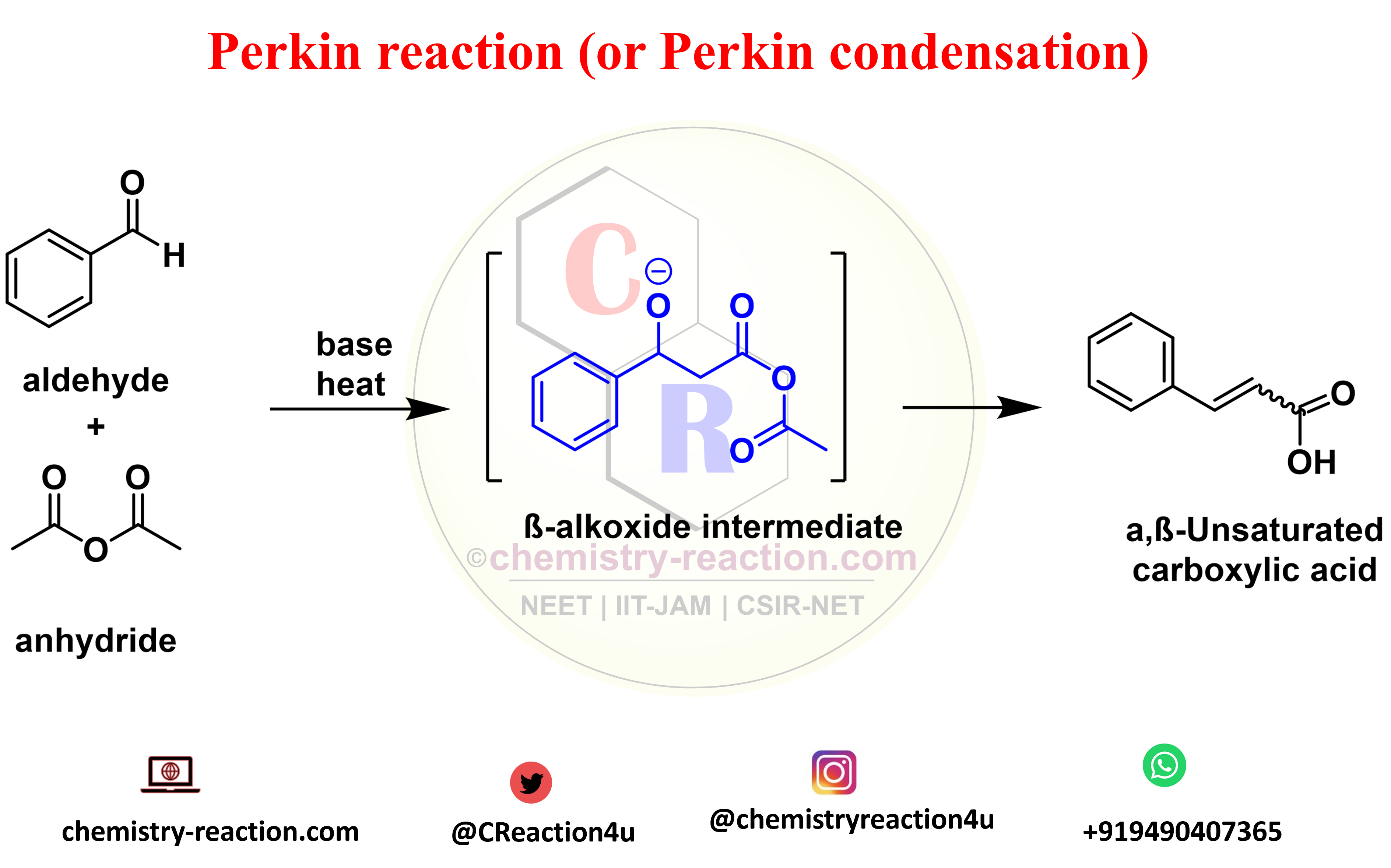
Suzuki cross-coupling Reaction: Examples | Mechanism | Application|
Suzuki cross -coupling reaction is an coupling reaction and also known as Suzuki-Miyaura cross-coupling, its coupling between a boronic acid and an organohalide or aromatic halides by using Palladium (0) complex is used to catalyst and base.