Table of Page Contents
What is Sandmeyer Reaction explain?
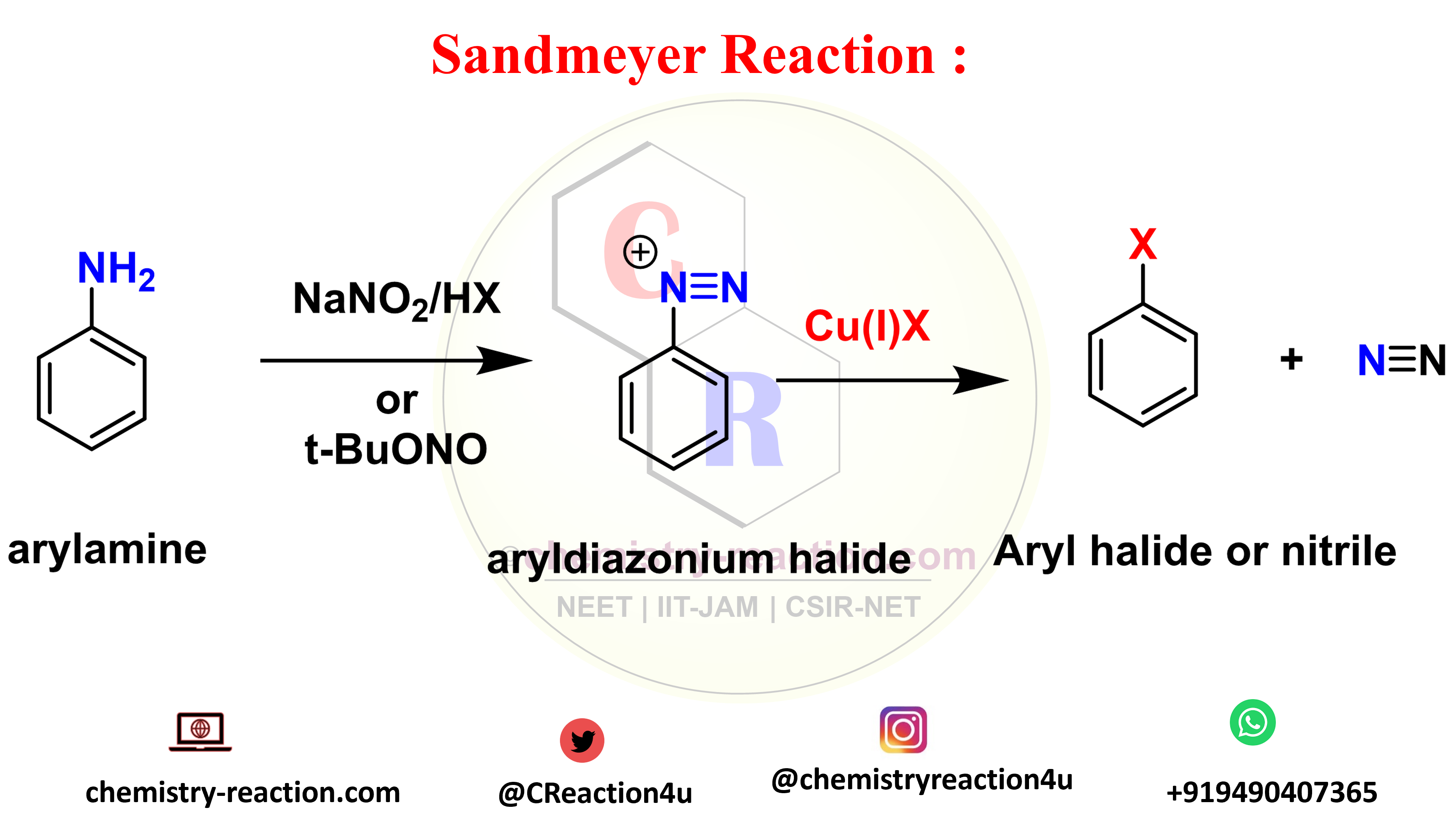
The Sandmeyer reaction is an organic substitution type reaction used for the synthesis of aryl halide from an aryl diazonium salt. the catalyst used in Sandmeyer reaction is a copper(I) halide like chloride, bromide or iodide ions catalyst. significantly, The reaction include trifluoromethylation, hydroxylation, cyanation, and halogenation type reaction on benzene. sandmeyer reaction mechanism explanation here.
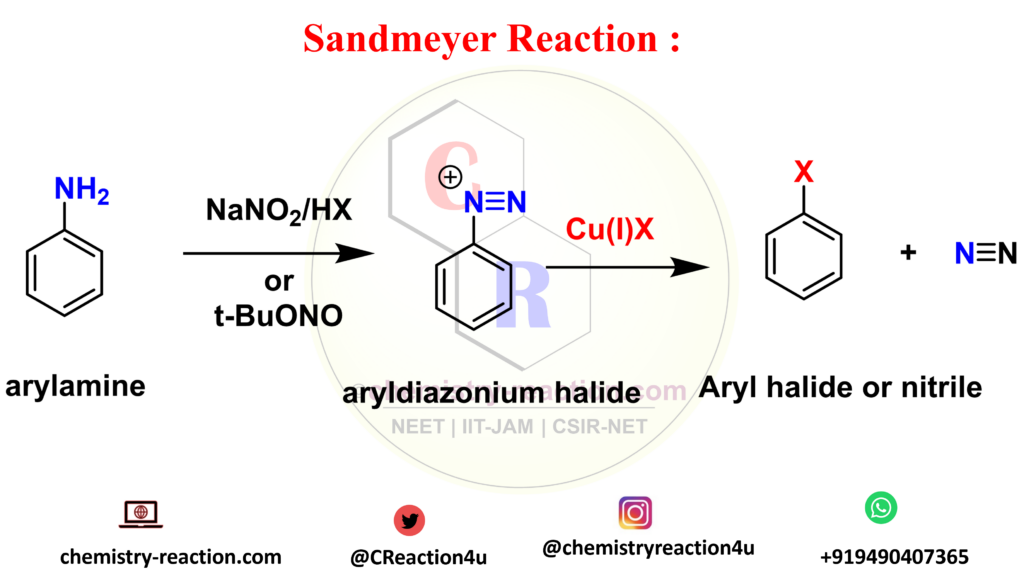
Sandmeyer Reaction Mechanism:
sandmeyer reaction mechanism explanation
- formation of nitronium ion from nitric acid
- Benzenediazonium Ion Formation
- formation of nitronium ion
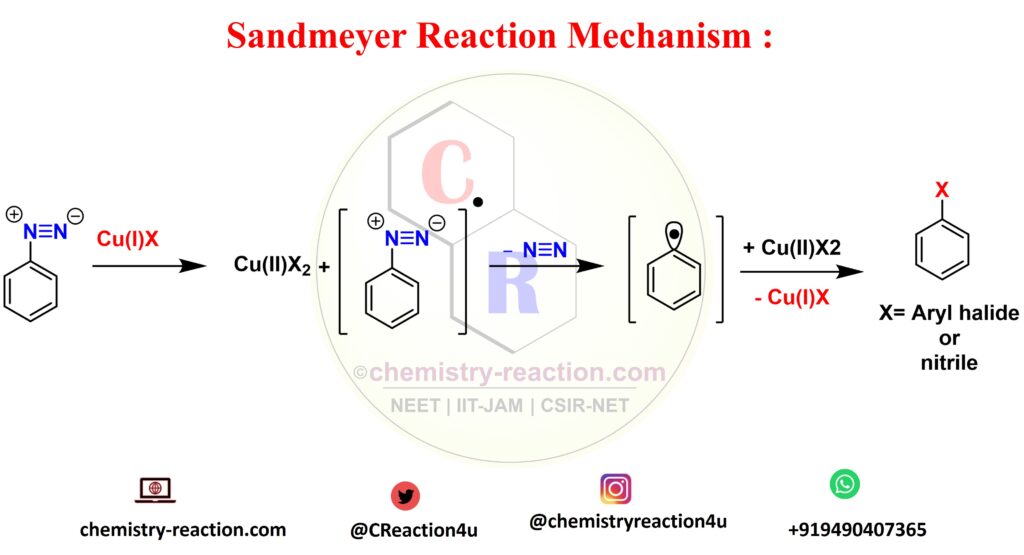
The Sandmeyer Reaction mechanism is two step mechanism, the first formation of nitronium ion from nitric acid mechanism second, Benzenediazonium Ion Formation from reaction of primary aniline and nitronium ion, and last free radical mechanism for synthesis of aryl halide.
looking into fist mechanism of formation of nitronium ion will take place by reacting sodium nitrate with acid, then primary amide nitrogen will attack and you can see the Benzenediazonium Ion Formation mechanism in picture below for more clarity. further next step is going to happen is ransfer of a single electron from the copper to the diazonium and its going to produce copper(II) halide and non-participating diazo radical by liberating nitrogen gas. the last is aryl radical will react with he copper(II) halide to regenerate the copper(I) halide catalyst and yield the final aryl halide product.
Importance of Sandmeyer Reaction:
sandmeyer reaction Example and Application:
- synthesis of benzonitriles by cyanation process
- synthesis of neoamphimedine (topoisomerase II as an anti-cancer drug)
- To create aryl halides.
Related reactions:
- Balz-Schiemann Reaction
- Hunsdiecker Reaction
References:
- Griess, J. P. Philos. Trans. R. Soc. London 1864, 164, 693.
- Sandmeyer, T. The substitution of the amine group with chlorine atom in aromatic systems. Ber. Dtsch. Chem. Ges. 1884, 17, 1633-1635
- Hanson, P., Rowell, S. C., Walton, P. H., Timms, A. W. Org. Biomol. Chem. 2004, 2, 1838-1855
- Kochi, J. K. The mechanism of the Sandmeyer and Meerwein reactions. J. Am. Chem. Soc. 1957, 79, 2942-2948.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

2 thoughts on “Sandmeyer Reaction: Definition| Mechanism| Example| Application”