This reaction named after W.H. Perkin, He described the synthesis of coumarin in one-pot by heating the salt sodium salicylaldehyde in acetic anhydride.
Table of Page Contents
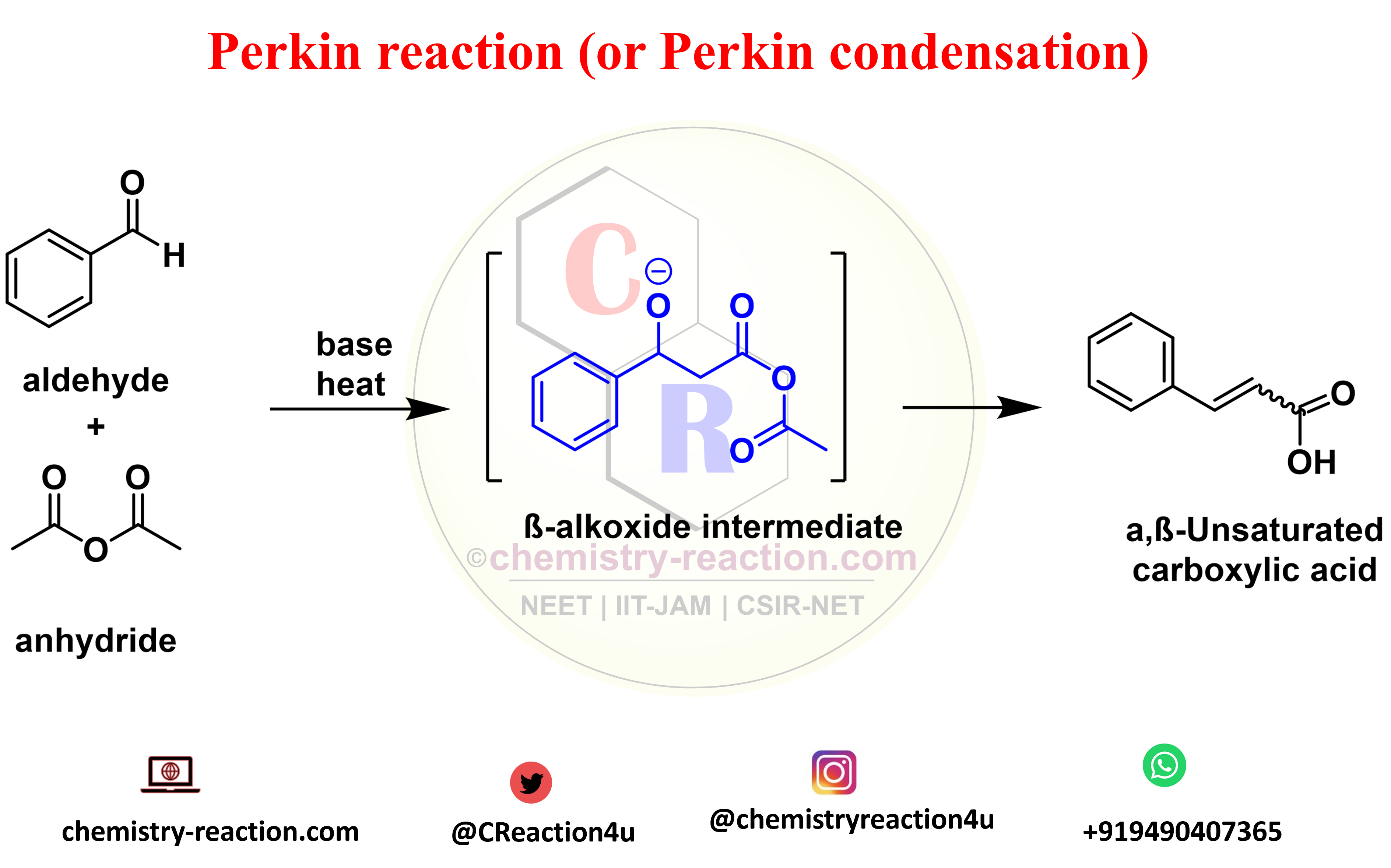
What is Perkin reaction (or Perkin condensation)?
The Perkin reaction (or Perkin condensation) is an condensation of aromatic aldehydes and the anhydrides of aliphatic carboxylic acids in the presence of a weak base to obtain α,β-unsaturated carboxylic acids.
the catalyst used in Perkin reaction is acetic anhydrides and base: NaOAc, KOAc, CsOAc, Et3N, pyridine, piperidine, K2CO3, aliphatic aldehydes with no α-hydrogens as well as certain α,β unsaturated aldehydes can also be used. The final product of Perkin reaction is α,β-unsaturated carboxylic acids. Synthesis of coumarins is one of the application of Perkin reaction.
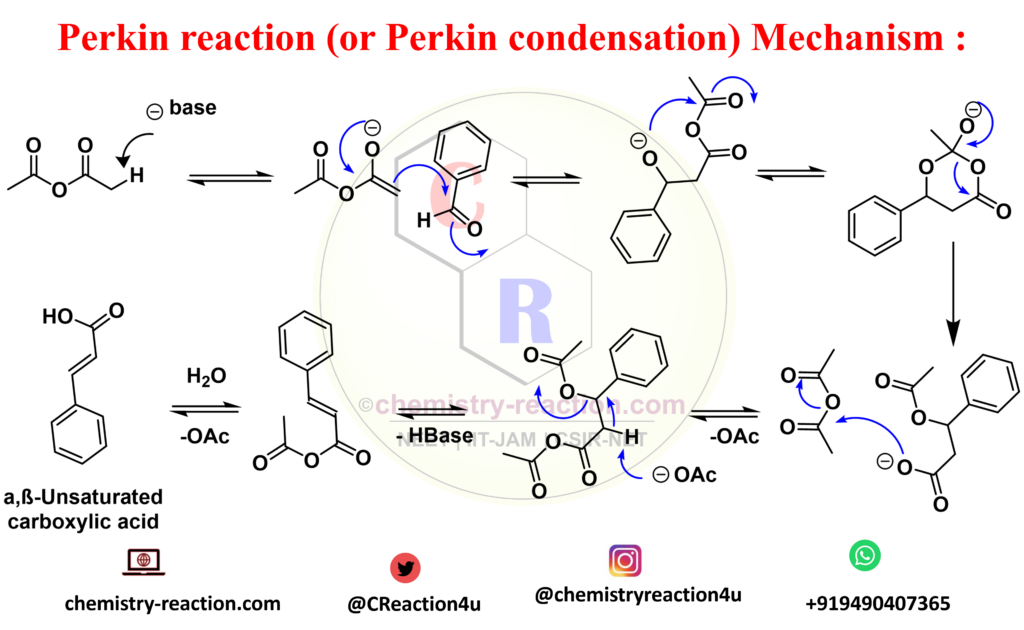
Perkin Reaction Mechanism:
Perkin Reaction examples and applications:
Related reactions :
- Pechmann condensation
- Stobbe condensation
References:
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.