Definition : In Dakin oxidation, aromatic aldehydes or ketones undergo treatment with RCO3H or H2O2 to smoothly oxidize them into carboxylic acids.
The reaction operates effectively when the aromatic aldehyde or ketone possesses electron-rich (-R, -OH, -OR, -NH2, or -NHR) substituents in the para or ortho positions. When the aromatic ring is substituted with electron-withdrawing groups, the resulting product of the oxidation typically comprises the carboxylic acid.
Reagents utilized in Dakin oxidation include Acidic H2O2, peroxybenzoic acid, peroxyacetic acid, sodium percarbonate, alkaline H2O2, and urea-H2O2 (UHP) adduct.
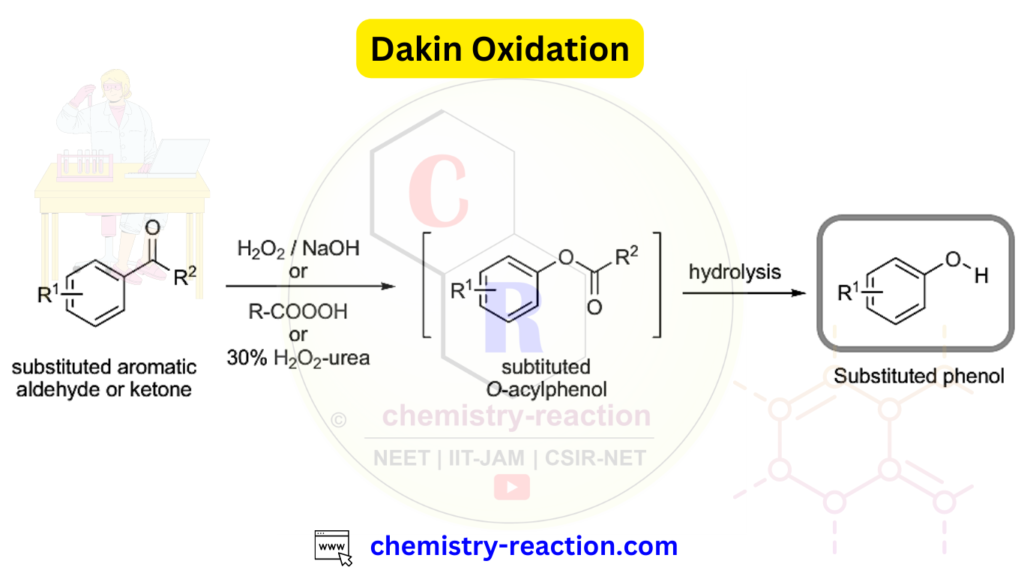
Table of Page Contents
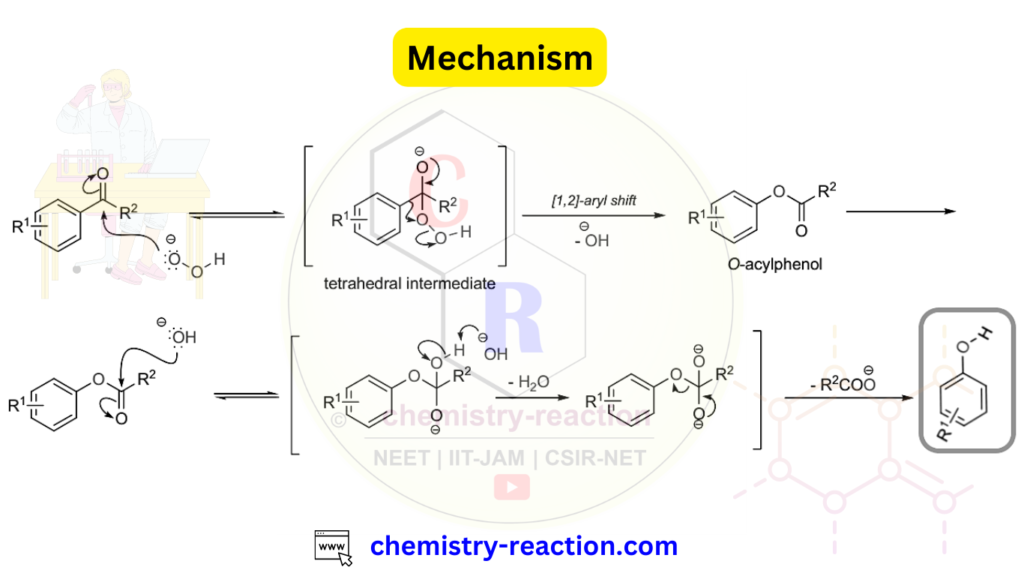
Dakin Oxidation Reaction Mechanism :
Dakin Reaction Mechanism: Under basic conditions (H2O2/NaOH), H2O2 is deprotonated to form HO-O-, which then adds across the carbonyl group of the aromatic ketone or aldehyde. The resulting tetrahedral intermediate undergoes a [1,2]-aryl shift, yielding an O-acylphenol. This O-acylphenol is subsequently hydrolyzed to produce the corresponding phenolate anion under the reaction conditions. Finally, during the work-up, the substituted phenol is liberated from the phenolate salt.
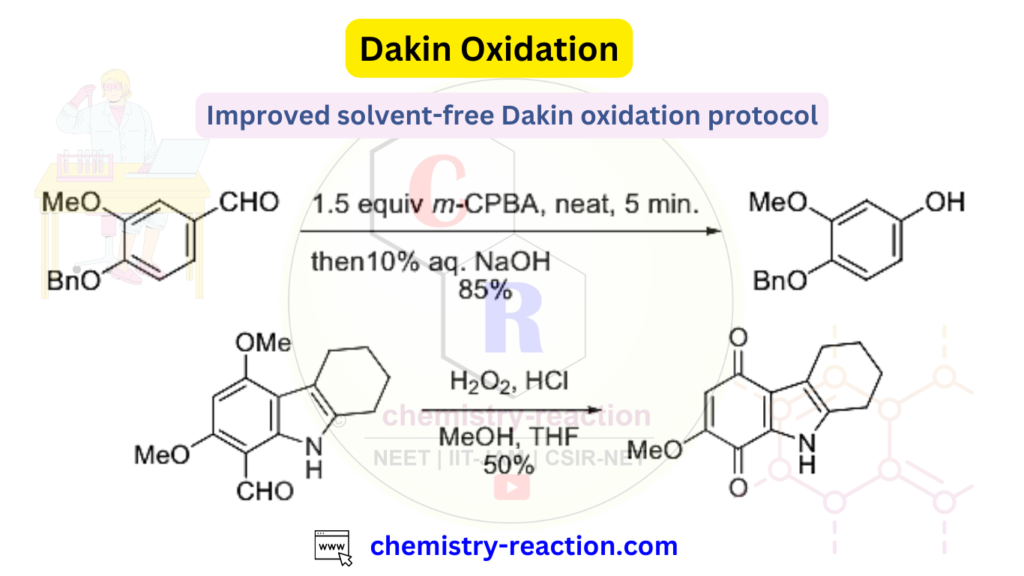
Dakin Oxidation Examples:
The improved solvent free Dakin oxidation protocol.
Dakin Oxidation Slideshare | Dakin oxidation Reaction | Dakin Oxidation ppt | Dakin Reaction | Dakin Oxidation Reaction Mechanism | Dakin Oxidation Notes| Dakin Oxidation pdf | Dakin Reaction Mechanism
Related reactions:
Reafference:
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “Dakin Oxidation Reaction : Definition | Mechanism | Examples”