The Aza-Claisen rearrangement is a chemical reaction involving a nitrogen atom’s migration from one position to another within a molecule. It is named after the Claisen rearrangement, a well-known rearrangement reaction involving oxygen atoms.
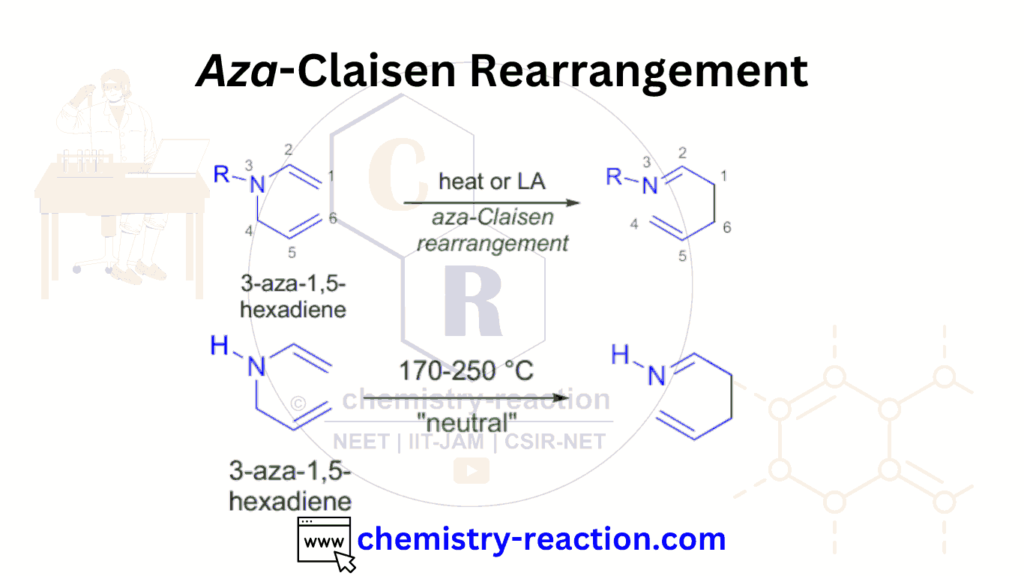
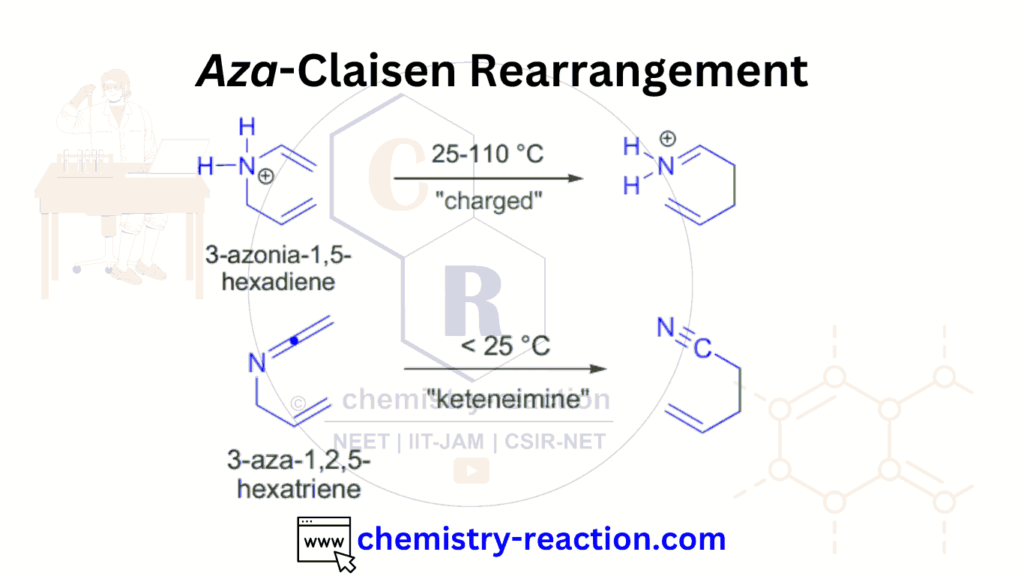
Table of Page Contents
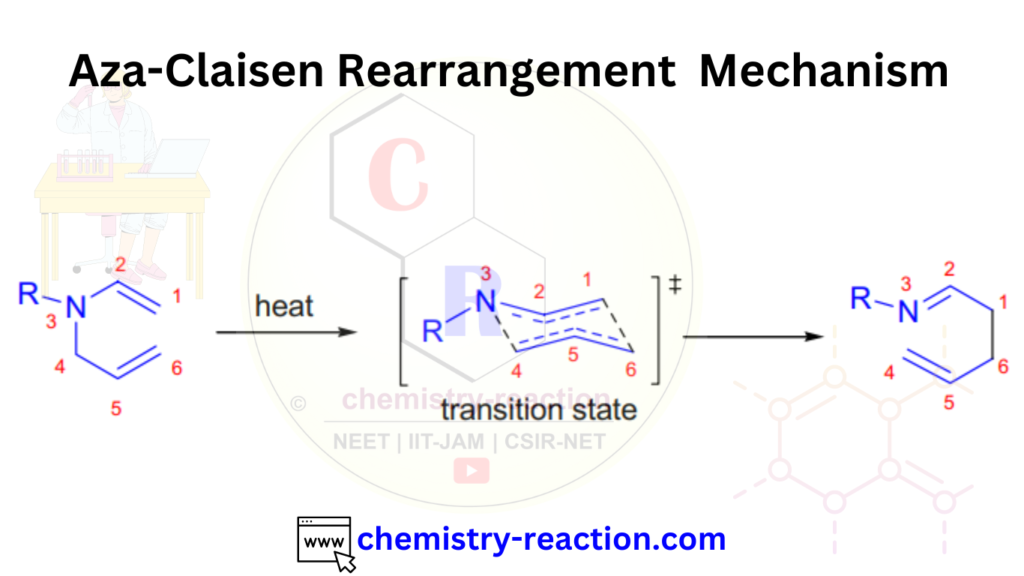
The mechanism of the aza-Claisen rearrangement:
The mechanism of the aza-Claisen rearrangement involves three steps:
Protonation: The reaction often starts with the protonation of a nitrogen atom within the molecule, make it more electrophilic.
Rearrangement: The nitrogen atom undergoes migration, typically through a series of bond-breaking and bond-forming steps, to a new position within the molecule. This migration can result in the forming of a new C-N bond.
Deprotonation: After the rearrangement, deprotonation may occur to regenerate the original nitrogen-containing functional group or to form a new functional group.
Aza- Claisen Rearrangement Examples:
These are the best examples of Aza Claisen rearrangements.
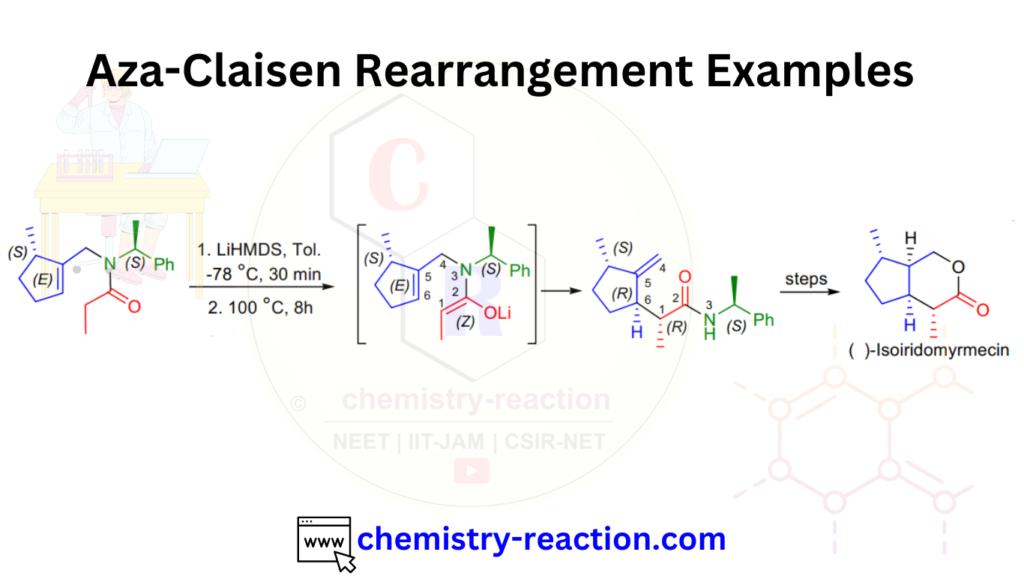
Aza- Claisen Rearrangement Examples
Related Reaction :
- Prins-Pinacol Rearrangement
- Lossen Rearrangement
- Pummerer Rearrangement
- Smiles Rearrangement
- Sommelet-Hauser Rearrangement
- Favorskii Rearrangement
- Brook Rearrangement
- Beckmann Rearrangement
- Baeyer-Villiger Oxidation/Rearrangement
- Aza-Cope Rearrangement
- Aza-Wittig Reaction
- Baker-Venkataraman Rearrangement
- Benzilic Acid Rearrangement
- Aza-[2,3]-Wittig Rearrangement
- Johnson-Claisen Rearrangement
- Eschenmoser-Claisen Rearrangement
- Claisen-Ireland Rearrangement
References:
Aza claisen rearrangement example, aza-cope rearrangement, claisen rearrangement pdf, application of claisen rearrangement, claisen rearrangement ppt
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “Aza-Claisen Rearrangement:”