Table of Page Contents
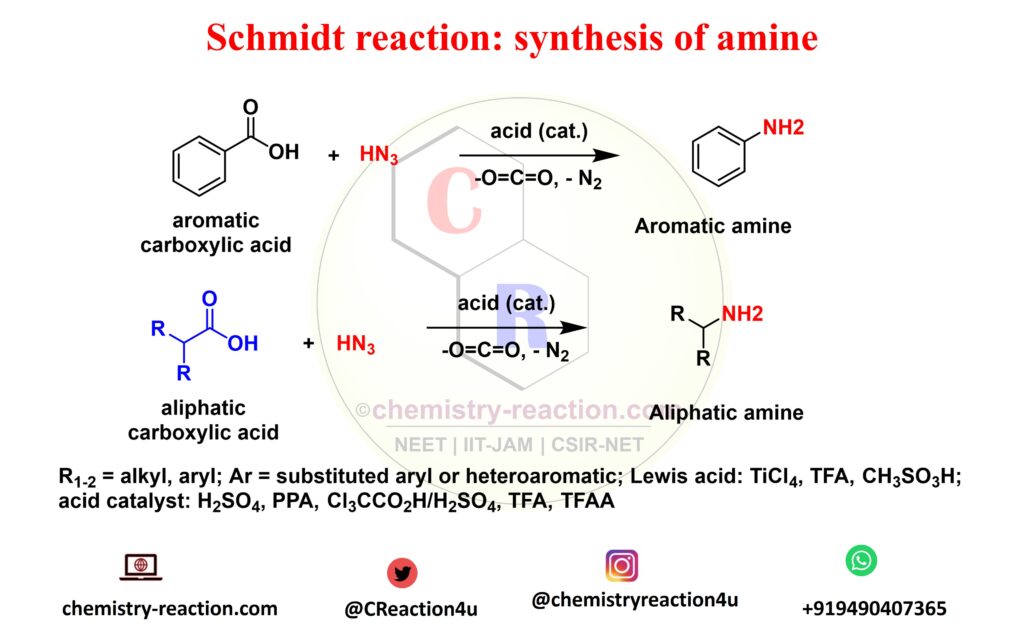
What is Schmidt Reaction explain?
Schmidt reaction is a acid-catalyzed Rearrangement reaction of hydrazoic acid reactions of electrophiles, like carbonyl compounds, alkenes tertiary and alcohols. The following these substrates include amines, nitriles, amides or imines, rearrangement and extrusion of nitrogen.

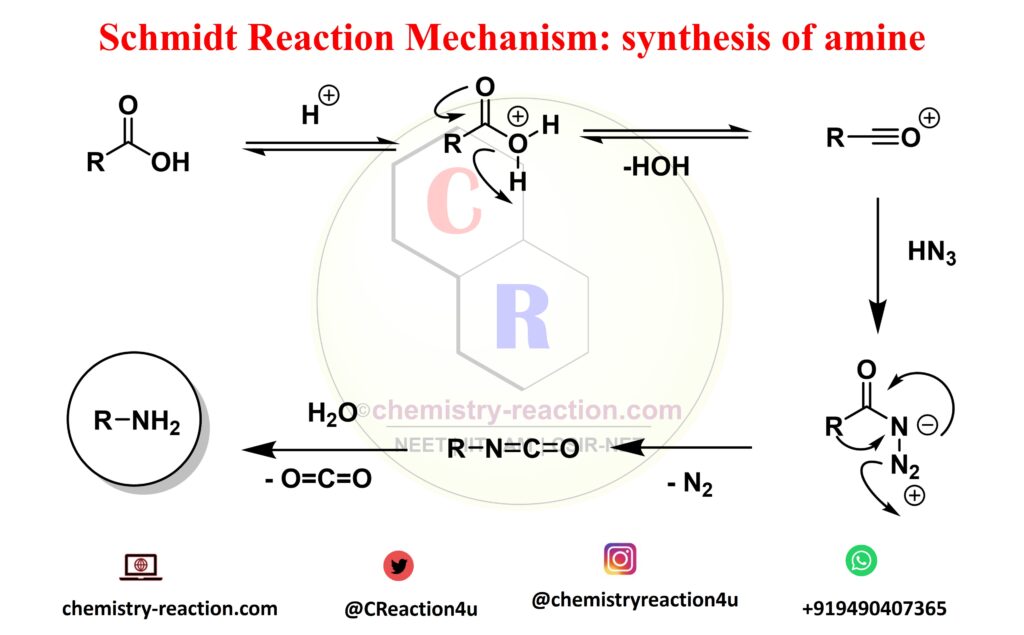
Schmidt Reaction Mechanism:
The mechanism shown is for an aldehyde regent but it can be easily extended to the other regents. The reaction begins with abstraction of a proton from the acid by the aldehyde or other reagent to activate it for future attack. The generated water molecule then abstracts the proton from hydrazoic acid to regenerate the acid and produce an azide anion which then attacks the activated reagent. A series of steps and the release of a molecule of nitrogen gas result in the final product.

Related Reaction:
| 1. Baeyer-Villiger oxidation | 2 Beckmann rearrangement |
| 3. Claisen rearrangement | 4. Cope rearrangement |
| 5. Curtius rearrangement | 6. Eschenmoser-Claisen rearrangement |
| 7. Fries rearrangement | 8. Hofmann rearrangement |
| 9. Ireland-Claisen rearrangement | 10. Johnson-Claisen rearrangement |
| 11. Wagner-Meerwein rearrangement | 12. Wolff rearrangement |
References:
- Schmidt, K. F. Z. Angew. Chem. 1923, 36, 511. Karl Friedrich Schmidt (1887–1971) collaborated with Curtius at the University of Heidelberg, where Schmidt became a Professor of Chemistry after 1923.
- Schmidt, K. F. Ber. dtsch. Chem. Ges. 1924, 57, 704–706.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.


1 thought on “Schmidt Reaction (Rearrangement): definition| Mechanism| example”