The Carroll Rearrangement is a named reaction in organic chemistry, where the [3,3]-sigmatropic rearrangement of allylic β-keto esters to form γ,δ-unsaturated ketones is called the Carroll rearrangement. It is also known as the Kimel–Cope or decarboxylative Claisen rearrangement. Here, we will explore the Carroll rearrangement and its mechanism, accompanied by some interesting examples.
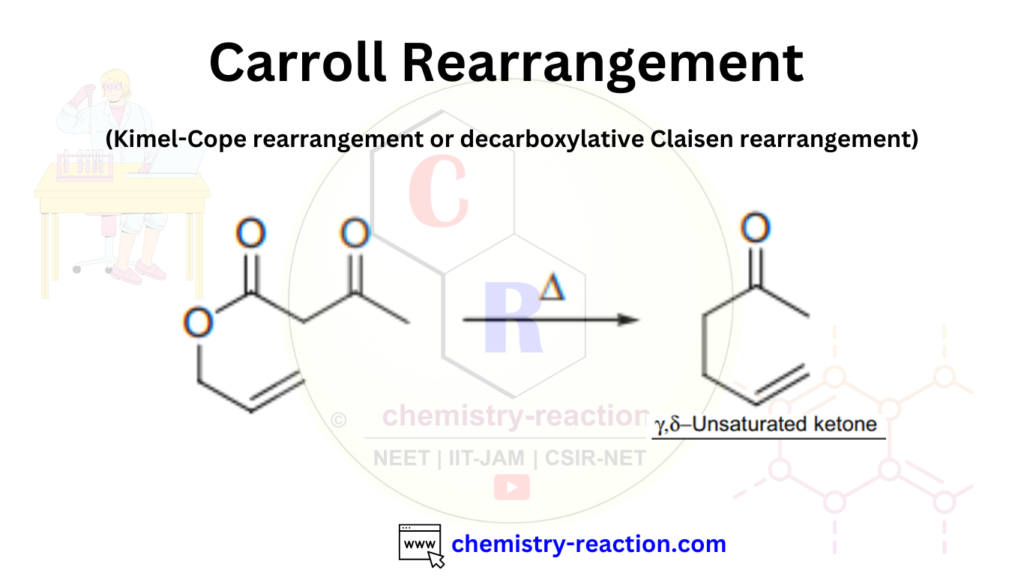
Table of Page Contents
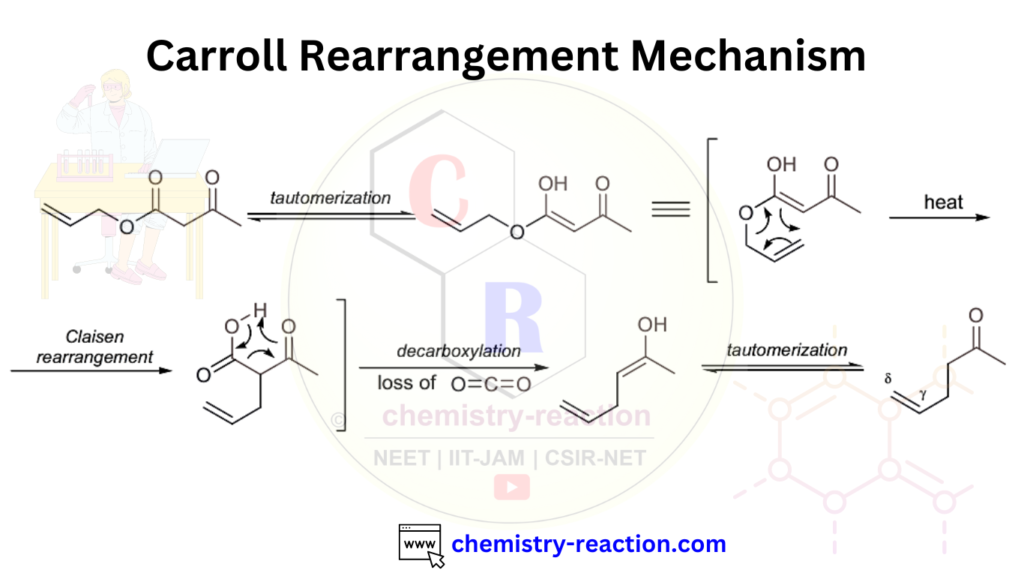
Carroll Rearrangement Mechanism:
Here is the stepwise mechanism of Carroll Rearrangement
- In first step an allylic β-keto ester generates its enol (or enolate) form.
- Second step: The enol undergoes a [3,3]-sigmatropic rearrangement (Claisen-type shift) through a six-membered cyclic transition state.
- This rearrangement gives an allyl β-keto acid intermediate.
- The β-keto acid is unstable and decarboxylates, releasing CO₂.
- Tautomerization of the resulting enol yields the γ,δ-unsaturated ketone.
Carroll Rearrangement Examples:
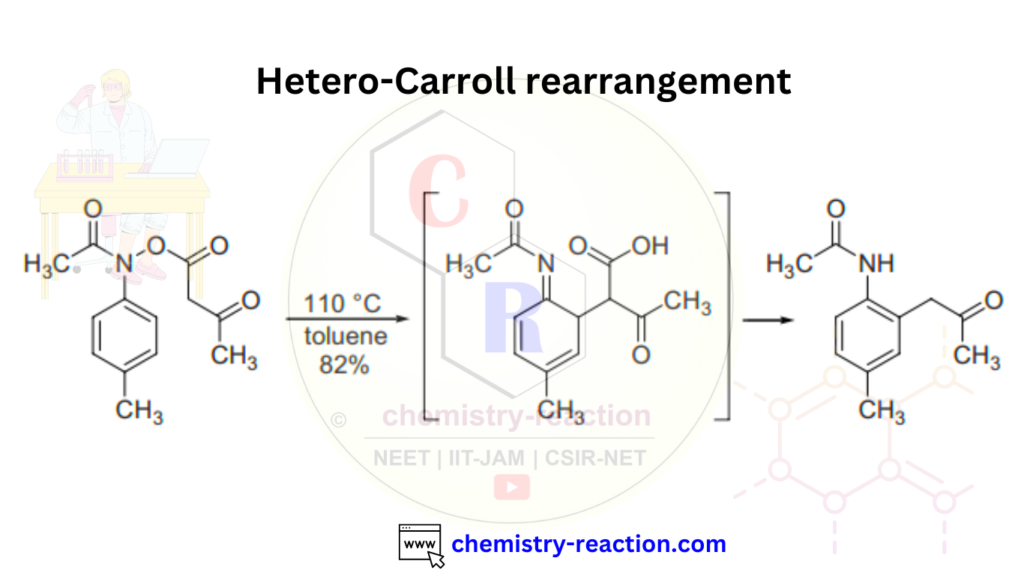
Hetero Carroll rearrangement is another version of Carroll rearrangement in organic chemistry.
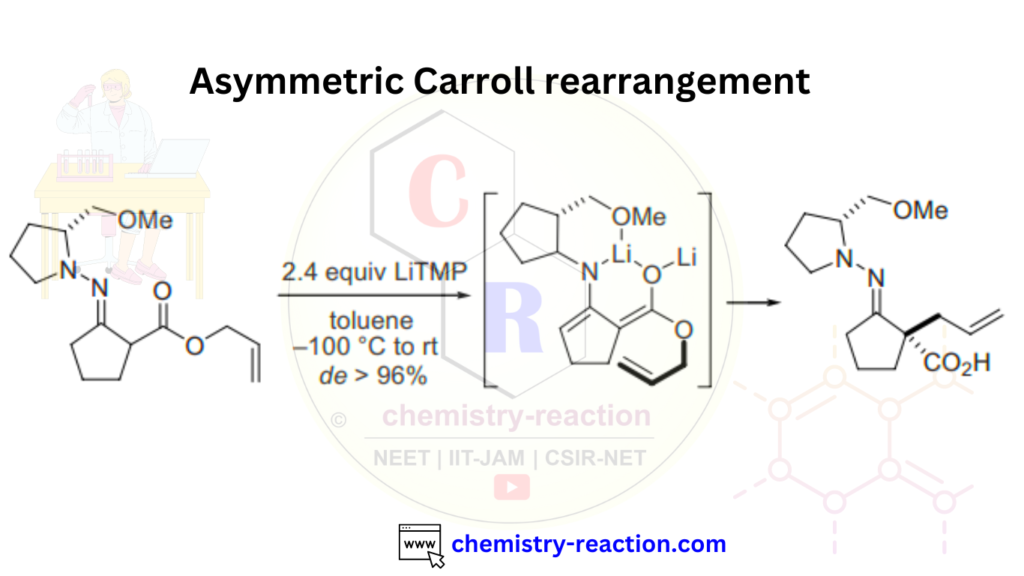
Here is an example of an asymmetric Carroll rearrangement reaction always used in organic chemistry.
Related Reactions:
- Oxy-Cope rearrangement
- Claisen rearrangement
- Cornforth rearrangement
- Ireland-Claisen rearrangement
- Ester rearrangement
References :
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
