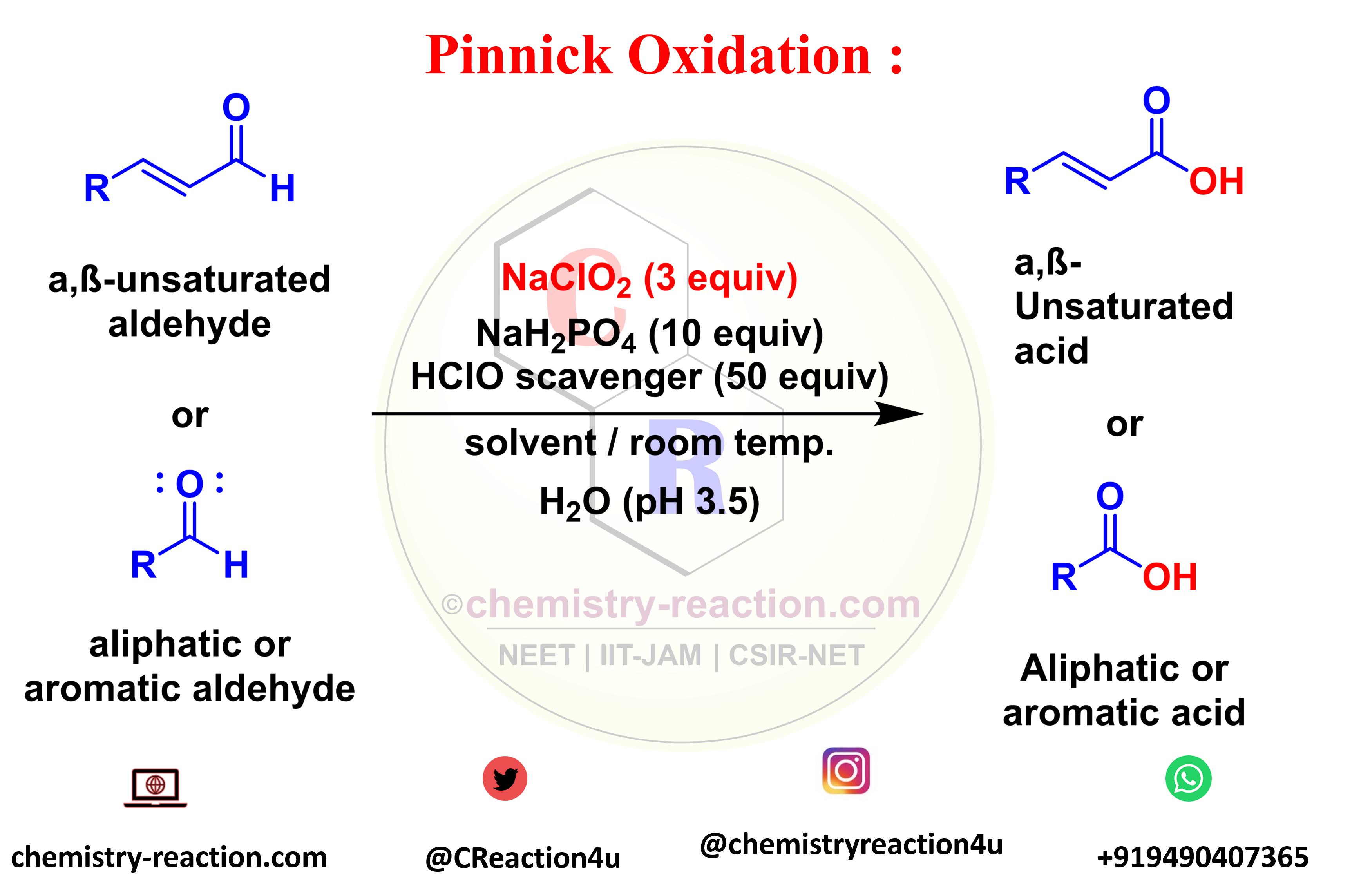
Pinnick oxidation
The transformation of aldehydes (aliphatic, aromatic, saturated, or unsaturated) to the corresponding carboxylic acids is known as the Pinnick Oxidation. The Pinnick oxidation procedure is the NaClO2/2-methyl-2-butene the system was generally used to oxidize a wide range of α and β-unsaturated Aldehydes without affecting any of the double bonds present in the substrate.