Burgess Reagent Mechanism, E.M. Burgess discovered that 2o and 3o alcohols could be dehydrated with the inner salt of (methoxycarbonylsulfamoyl)triethylammonium hydroxide the reagent is called the Burgess reagent to afford the corresponding olefins through intramolecular elimination reaction. This process is now known as the Burgess dehydration reaction.
Table of Page Contents
Burgess Reagent:
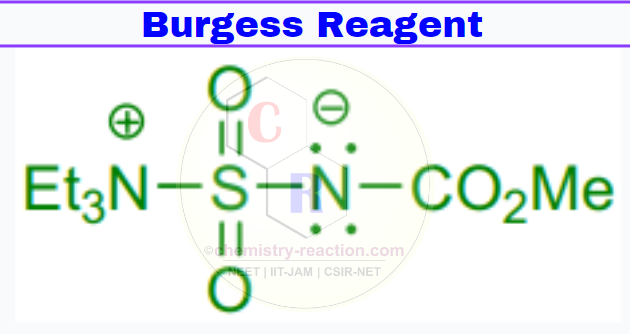
Burgess Reagent cas : 29684-56-8
Synonym(s): Methyl N (triethylammoniosulfonyl)carbamate,
Burgess Reagent Preparation:
The Burgess reagent (Burgess Reagent Amide Martin Sulfurane) is synthesized from chlorosulfonylisocyanate by reaction with triethylamine and methanol in a benzene solvent.
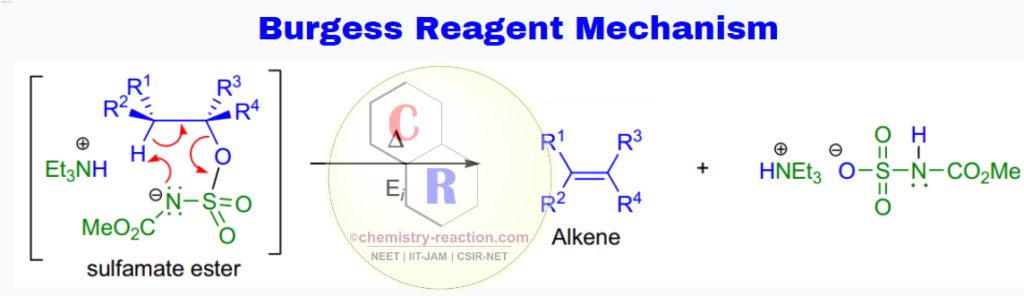
Burgess Reagent Mechanism:
The mechanism involves a stereospecific syn-elimination via ion-pair formation from the intermediate sulfamate ester (comparable to the Chugaev elimination of xanthate esters). Kinetic and spectroscopical data are consistent with an initial rate-limiting appearance of an ion-pair followed by a fast cis-β-proton transfer to the departing anion.
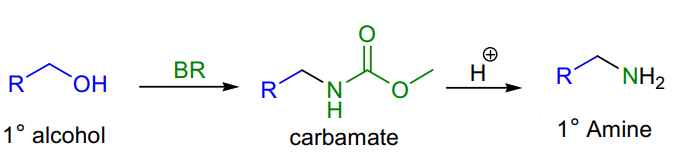
Uses :
- Primary alcohols are converted to the corresponding carbamates, giving primary amines after hydrolysis.
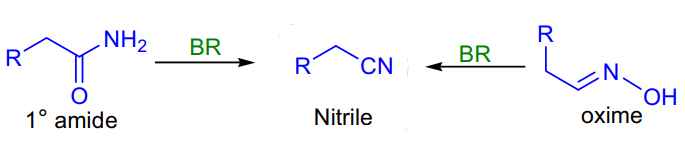
- Burgess Reagent Amide to Nitrile: dehydrating primary amides and oximes.
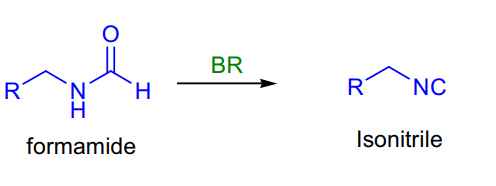
- Formamide to isonitrile
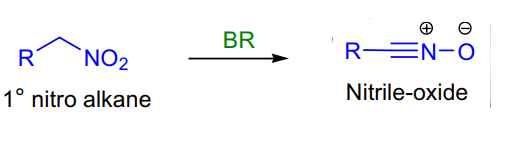
- Primary nitro alkanes to nitrile oxides synthesis
Burgess Reagent Oxidation:
Related reaction :
- Chugaev elimination
- Cope elimination
- Hofmann elimination
- Takai Reaction
References :
- The reactions of an N-sulfonylamine inner salt – Click
- Thermal reactions of alkyl N-carbomethoxysulfamate esters – Click
- Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms – J. Chem. Educ. 2005, 82, 12, 1780
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.


1 thought on “Burgess Reagent Mechanism | Burgess Dehydration Reaction”