The Arndt-Eistert reaction is a organic name reaction that converts a carboxylic acid into an N-acyl homologue of an amino acid. It involves the treatment of a carboxylic acid with diazomethane to generate a methyl ester, followed by reaction with a halogen to introduce an acyl group, producing the N-acyl derivative of the original carboxylic acid. The reaction is named after the German chemists Fritz Arndt and Bernd Eistert, who first reported it in 1935.
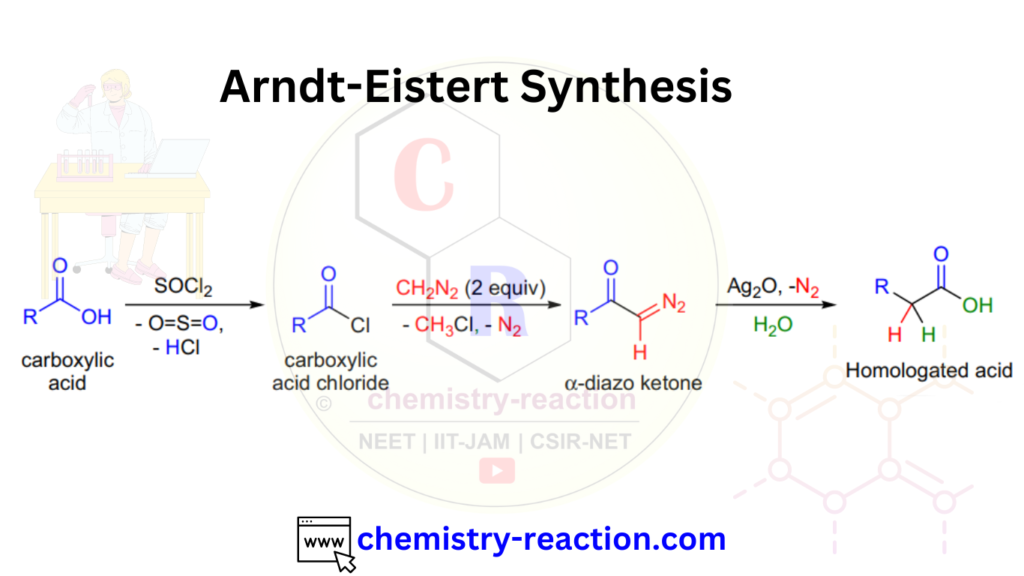
Arndt-Eistert Reaction Mechanism:
The Arndt-Eistert reaction proceeds through the following stepwise mechanism:
- Formation of diazomethane: The reaction begins with the generation of diazomethane (CH2N2) from nitrosomethylurethane or another precursor.
- Conversion of carboxylic acid to methyl ester: The diazomethane is then used to convert the carboxylic acid to its methyl ester by an insertion reaction. The carbenoid intermediate formed in this step is highly reactive and can potentially react with other functional groups in the reaction mixture, which makes the reaction somewhat unpredictable.
- Halogenation: The next step involves halogenation of the methyl ester, typically with chlorine or bromine, to form an acyl halide. This is usually accomplished using a halogenating agent such as phosphorus pentachloride (PCl5) or thionyl chloride (SOCl2).
- Substitution with an amine: Finally, an amine is added to the reaction mixture, which undergoes nucleophilic substitution with the acyl halide to form the N-acyl derivative of the original carboxylic acid.

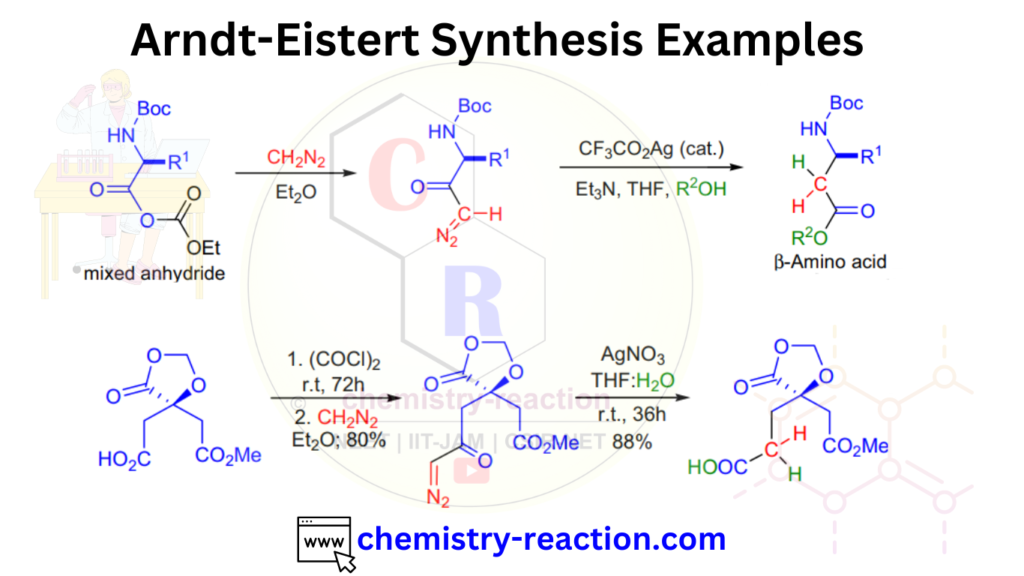
Overall, the Arndt-Eistert synthesisis a very useful method for the synthesis of N-acyl amino acid derivatives, which have applications in peptide synthesis and pharmaceutical chemistry.
Related Reactions:
- Wolff Rearrangement
- Homologations
- Kowalski Ester Homologation
- Synthesis of carboxylic acids
- Synthesis of α-Diazoketones
Reference:
- Arndt-Eistert Synthesis – OCP
- Arndt-Eistert Homologation – sciencedirect
- Diazomethane Synthesis and Applications – ProfessorDaveExplains
Here are all Name Reactions .
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
