Table of Page Contents
what is Swern Oxidation ?
The oxidation of primary and secondary alcohols using DMSO and TFAA or oxalyl chloride is known as Swern oxidation.
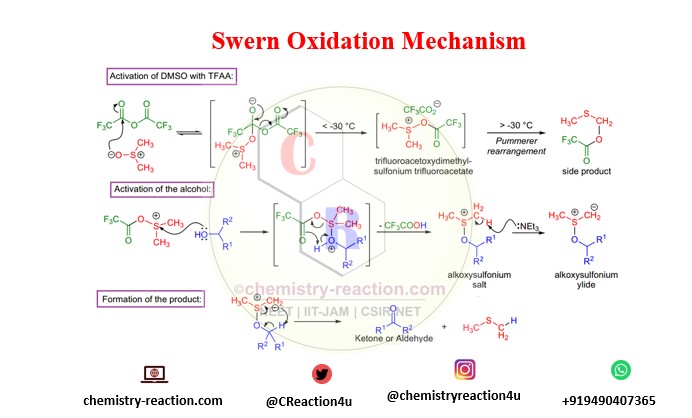
Swern oxidation reported in 1976, that treating DMSO with trifluoroacetic anhydride (TFAA) below -50 °C in methylene chloride gave trifluoroacetoxydimethylsulfonium trifluoroacetate, which reacted rapidly with primary and secondary alcohols. The resulting alkoxydimethylsulfonium trifluoroacetates, upon addition of triethylamine, afforded the subsequent aldehydes and ketones.
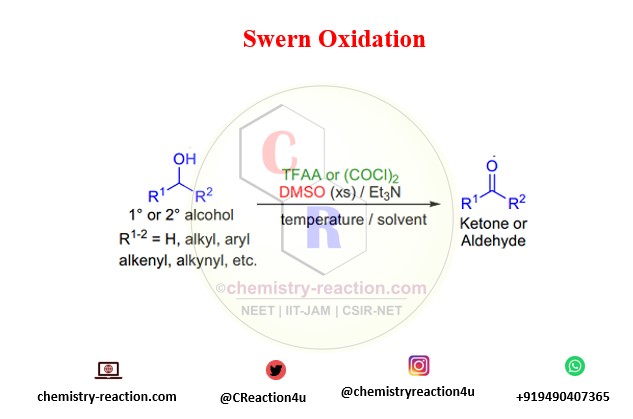
Oxalyl chloride was observed to be more successful than trifluoroacetic anhydride as a stimulating agent for DMSO in the oxidation of alcohols.
The common features and procedure of Swern oxidation:
- When no solvent is used, DMSO reacts violently with trifluoroacetic anhydride or oxalyl chloride, so great care should be exercised while running the reaction, so the common solvent is DCM.
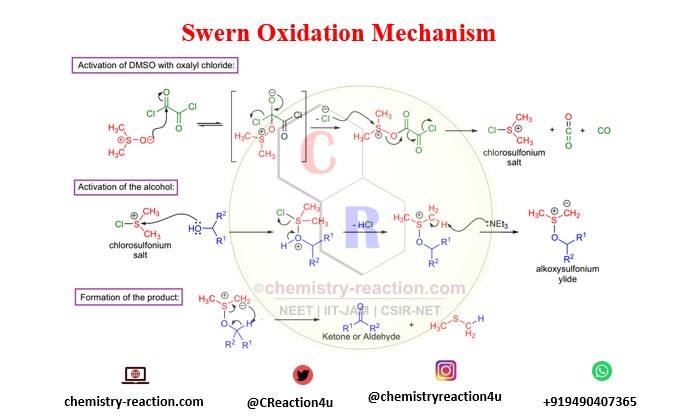
- If TFAA is used, the early intermediate is unpredictable above -30 °C, and a side product is formed via the Pummerer rearrangement. And if oxalyl chloride is utilized, the initial arbitrator is unpredictable above -60 °C, so the oxidation is typically conducted at -78 °C.
- The standard procedure was set up with the reaction of DMSO with TFAA or oxalyl chloride at below -30 °C temperature followed by the slow addition of the alcohol, then a tertiary amine.
- The steric hindrance of the substrate will not influence the efficiency of the oxidation.
- The use of trifluoroacetic anhydride might give increase to trifluoroacetate side products, whereas in the case of oxalyl chloride side reactions are exceptional.
Mechanism of Swern Oxidation:
Swern Oxidation mechamism for oxidation of alcohol by using Trifluoroacetic anhydride. swern oxidation mechanism primary and secondary alcohol.
Swern Oxidation mechamism for oxidation of alcohol by using oxalyl chloride. swern oxidation mechanism primary and secondary alcohol.
Applications of Swern oxidation Reaction:
swern oxidation produces byproducts: Dimethyl sulfide (Me2S), also known as DMS), Carbon monoxide (CO), Carbon dioxide (CO2),
Related Reaction:
- Dakin oxidation
- Criegee Oxidation
- Jones Oxidation
- Ley Oxidation
- Pinnick Oxidation
- Riley Selenium Dioxide Oxidation
- Rubottom Oxidation
- Corey-Kim oxidation
- Lieben haloform reaction
- Oppenauer oxidation
- Pfitzner-Moffatt oxidation (Moffatt oxidation)
- Saegusa oxidation
References :
- https://www.organic-chemistry.org/namedreactions/swern-oxidation.shtm
- https://chem.libretexts.org
- https://www.sciencedirect.com/topics/chemistry/swern-oxidation
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
