Table of Page Contents
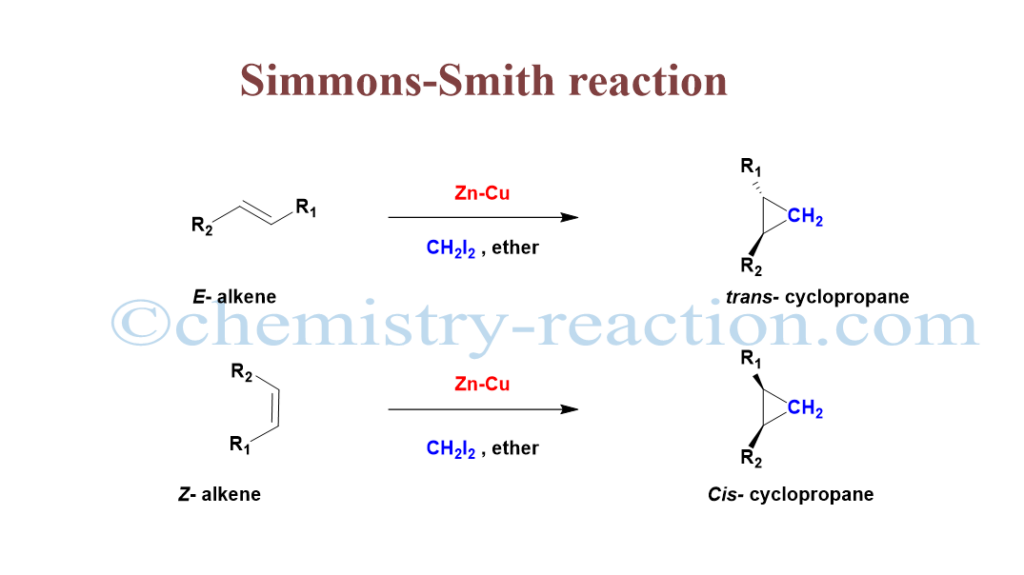
What is Simmons–Smith Reaction?
Simmon Smith reaction is most powerful organic cheletropic (reaction) method for cyclopropane preparation from diiodomethane (CH2I2) in the presence of zinc-copper couple (Zn-Cu) from unfunctionalized alkenes (e.g., cyclohexene, styrene) stereospecifically.
This method is highly stereocontrolled, and it involves a C=C bond-breaking reaction and the addition of the CH2 group. For example, if the alkyl groups of the alkene are trans-alkenes, give trans-cyclopropanes, and cis- alkene gives cis- in the cyclopropane.
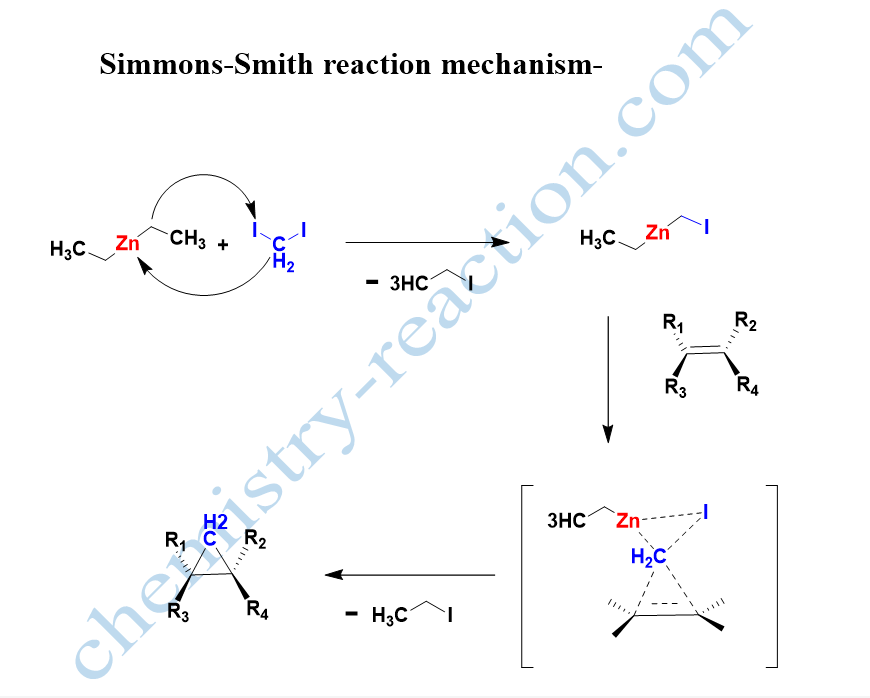
Simmon Smith Reaction Mechanism –
The number of reactions, theoretical studies, and the stereochemical outcome show that the Simmon Smith reaction mechanism is a concerted process. It proceeds via a three-centered “butterfly-type” type transition state.
simmons smith reaction intermediate is methylene free radical intermediate. simmons smith reaction is stereospecific to the alkene.
Additional Information –
- Simple alkenes, α,β-unsaturates ketones and aldehydes, electron-rich alkenes (enol ethers, enamines).
- More electron-rich alkenes will react faster bcz the electrophilic nature of the reagent.
- Highly substituted alkenes can react slowly bcz of steric hindrance.
- The cyclopropanation is diastereoselective, and it occurs less hindered face of the double bond.
- When alkene substrate has OH, OAc, OMe, OBn, and NHR functional groups, they act as directing groups for delivering stereochemical outcomes.
Related Reactions:
References:
- E. Lévesque, S. R. Goudreau, A. B. Charette, Org. Lett., 2014, 16, 1490-1493.
- A. Voituriez, L. E. Zimmer, A. B. Charette, J. Org. Chem., 2010, 75, 1244-1250.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

