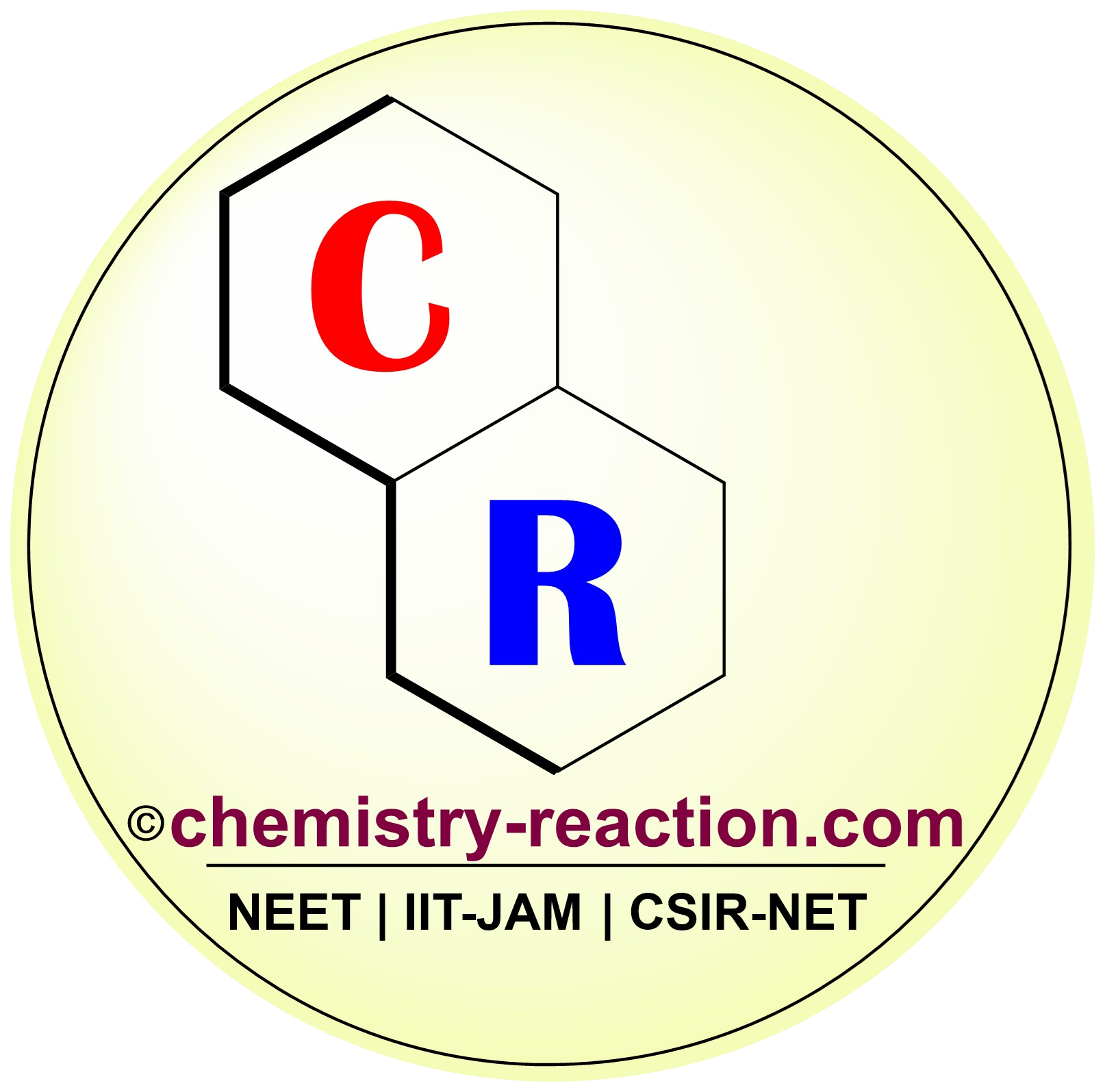
The Reimer-Tiemann reaction is an organic transformation that converts a phenol into an 2-hydroxy benzaldehyde using chloroform, a base, and an acid work-up at last step.
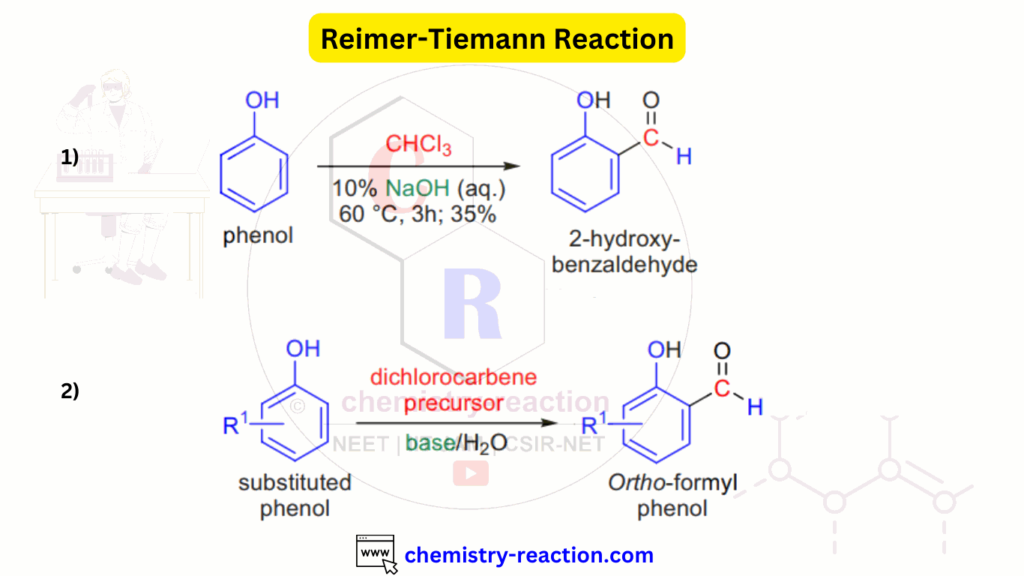
Table of Page Contents
Reimer-Tiemann Reaction Mechanism:
The reaction commences with removing a proton from chloroform by the base, forming a trichlorocarbanion. This intermediate, however, spontaneously eliminates a chloride ion, generating a neutral dichlorocarbene. Simultaneously, the base deprotonates the phenol reagent, rendering it reactive. The deprotonated phenol then proceeds to attack the dichlorocarbene. Subsequent sequential steps, followed by an acid work-up, form the desired o-hydroxy benzaldehyde product.
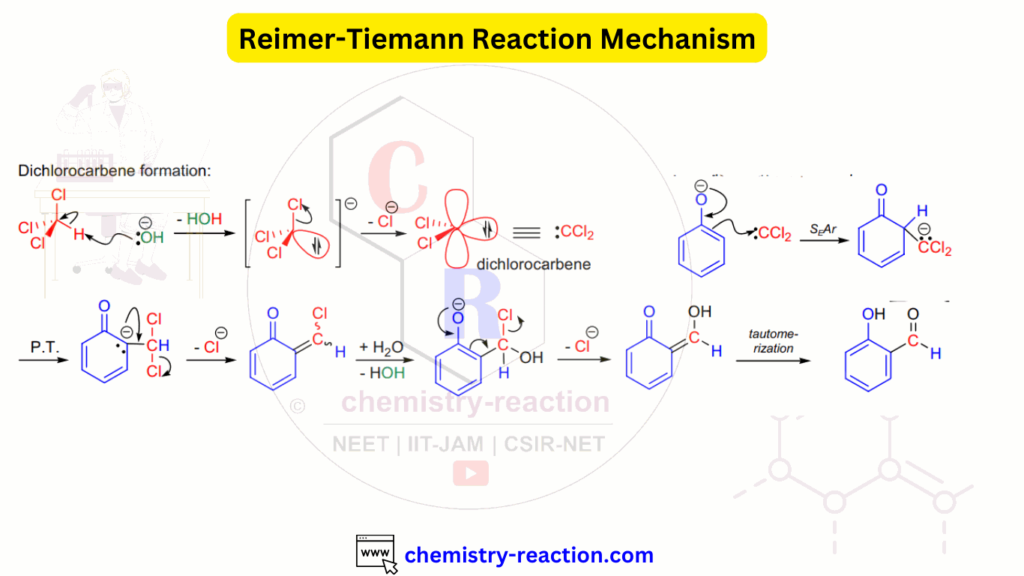
Reimer-Tiemann reaction product is o-hydroxy benzaldehyde. Reimer-Tiemann reaction of phenol is well known Name reaction in organic chemistry. neutral dichlorocarbene is intermediate of Reimer-Tiemann reaction.
Reimer Tiemann Reaction Application:
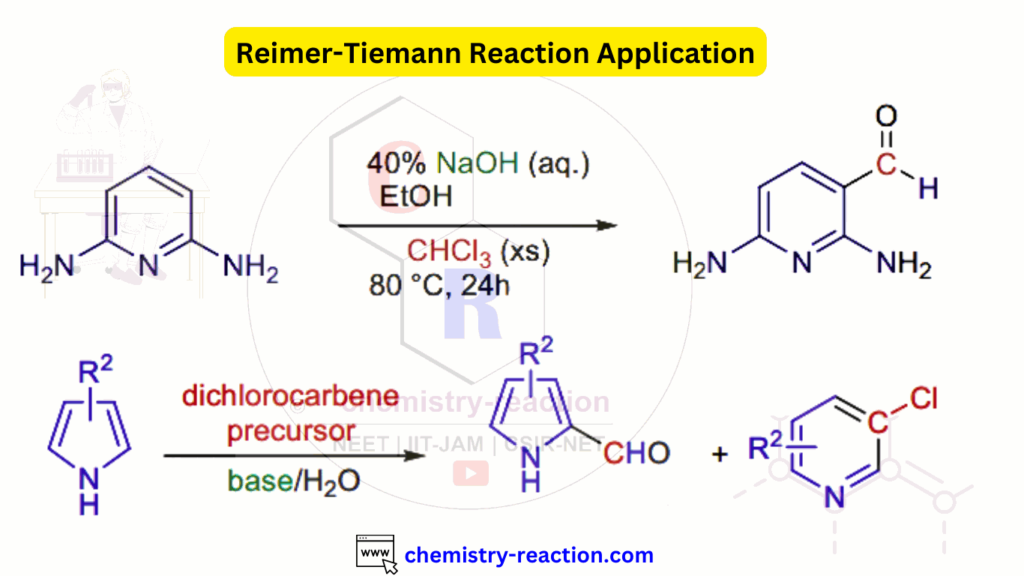
Reimer Tiemann Reaction Examples:
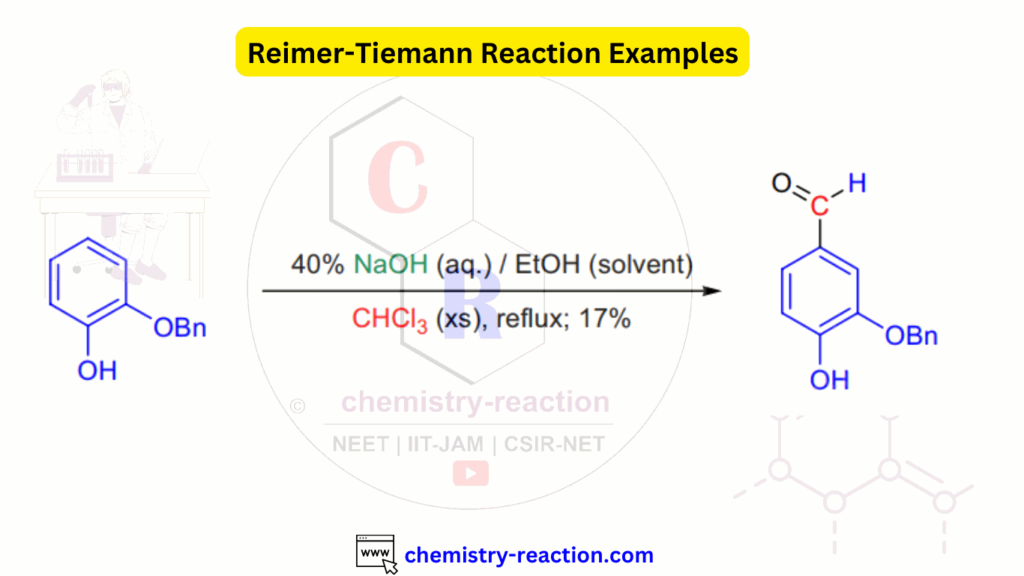
Related Reactions:
References:
- Reimer-Tiemann Formylation – sciencedirect
- The Reimer-Tiemann Reaction – pubs.acs
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.