Mannich Reaction PDF | Mannich Reaction Examples | Mannich Reaction Definition | Mannich Reaction Mechanism PDF | Mannich Reaction PPT | Mannich Reaction Procedure
The Mannich Reaction was invented in 1912 by German chemist Carl Mannich, and has been the subject of study, extensions, and applications ever since.
Table of Page Contents
Definition:
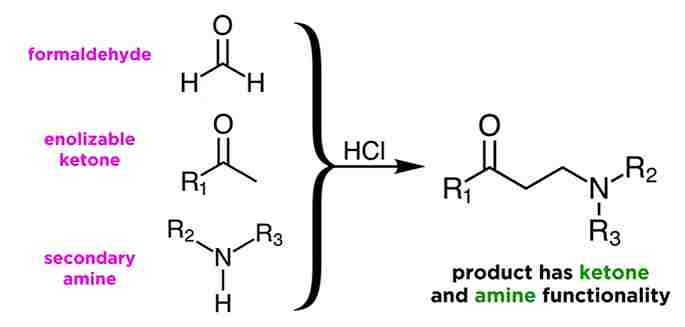
The condensation of a CH-activated compound usually an aldehyde or ketone with a primary or secondary amine or ammonia and a non-enolizable aldehyde or ketone to afford aminoalkylated derivatives is known as the Mannich reaction.
Explain Mannich Reaction:
Mannich Reaction utilizes three components, those formaldehyde, an enolizable ketone, and a secondary amine. The reaction is usually catalyzed by a mineral acid such as hydrochloric acid, and will yield a product like this, with both ketone and amine functionality. i.e a beta-amino carbonyl compound.
Mechanism of Mannich Reaction:
when formaldehyde is in the presence of a secondary amine, like dimethylamine, and under acid catalysis. As we can see the amine attacks the carbonyl, the resulting hydroxyl is protonated by the acid, and then dehydration takes place to form the iminium ion. This is also quite reactive as an electrophile, but reacts only once with ketone enols, and the reaction is usually quite clean.
Here we see the aldol-like reaction which occurs between the enol of the ketone and the iminium ion from the first sequence, which will yield the beta-amino carbonyl compound (mannich base). The acid catalyzes the equilibration between ketone and enol, and although at equilibrium the ketone is by far the predominant species, there is enough reactive enol present in solution to drive the transformation.
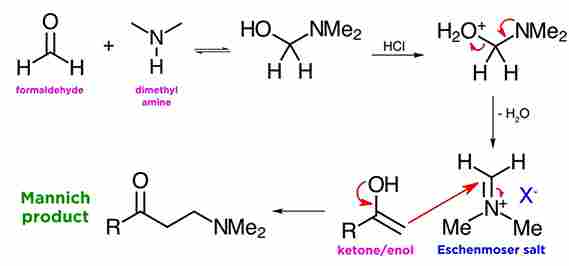
Eschenmoser salts:
Eschenmoser salts, is a like the iminium ion from before with some counterion, and these are called Eschenmoser salts, after their discoverer, the Swiss chemist Albert Eschenmoser. These Eschenmoser salts are often commercially available, although they are moisture sensitive and must be handled under a nitrogen atmosphere.

Synthetic Application of Mannich Reaction:
Here the few Mannich reaction application
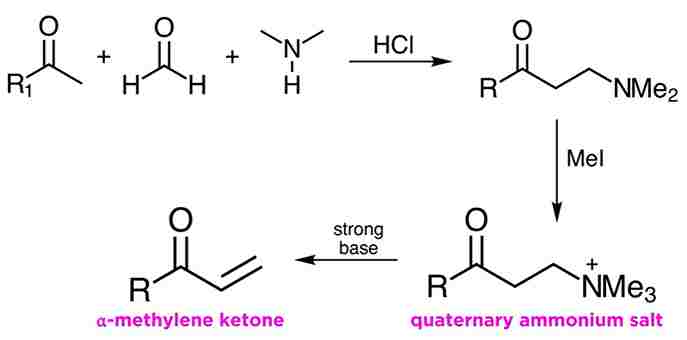
Formation of an α-methylene ketone
One of the more interesting applications of the Mannich reaction is in the methylenation of enolizable ketones. The Mannich product from a moment ago can be alkylated with methyl iodide, and this leads to a quaternary ammonium salt. This moiety is a good leaving group, as we have learned in a previous tutorial, and treatment with strong base leads to β-elimination and the formation of an α-methylene ketone.
Mannich Reaction of phenols and indoles :
Now for another application, Eschenmoser salts are very electrophilic, and do not react only with ketone enolates, but also with enolates derived from esters, nitriles, nitro compounds, and the like. Even electron-rich aromatics are very common nucleophilic partners in Mannich-like reactions. Here are some examples using phenols and indoles. This application will remind the observant chemist of the Friedel-Crafts reactions we learned about earlier in the series.

Stephen Aldehyde Synthesis, Mechanism and Application
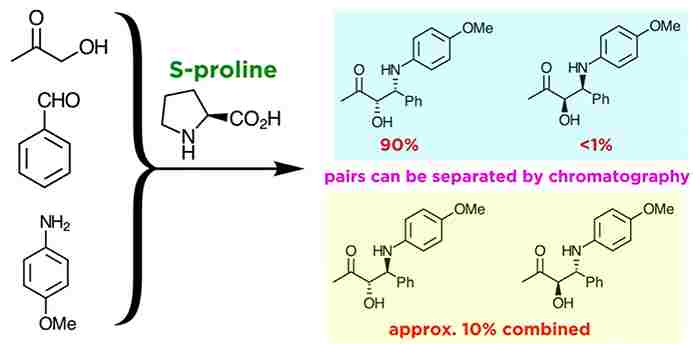
Asymmetric Mannich Reactions :
However, Asymmetric Mannich reactions show what fine level of control is possible with chiral catalysis, here is an example of a selective reaction under rather simple conditions. The amino acid S-proline is the catalyst responsible for the high level of stereocontrol. this synthesis allows for the preparation of an enantiomerically pure product. But for our purposes,
we now understand the basics regarding mannich reaction of ferrocene, mannich reaction examples, mannich reaction procedure, mannich reaction ppt. if you need ncert solutions for class 10 maths, class 12 chemistry ncert solutions please follow our website.
Related Reaction:
- Eschenmoser Methenylation
- Eschenmoser-Claisen Rearrangement
- Eschenmoser-Tanabe Fragmentation
- Petasis Boronic Acid-Mannich Reaction
- Betti reaction
- Kabachnik–Fields reaction
- Pictet–Spengler reaction
- Stork enamine alkylation
- Nitro-Mannich reaction
References :
- Organocatalyzed Asymmetric Mannich Reaction: An Update
- Professor Dave Explains
- Mannich Reaction – an overview
- Mannich reaction – Wikipedia
- Organic Chemistry Reagents Table
- Sakurai Allylation Reaction Mechanism with Applications
- Saegusa Oxidation Reaction Mechanism with Applications
- Smiles Rearrangement Reaction : Mechanism , Application and Modification
- Snieckus Directed Ortho Metalation Reaction
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “Mannich Reaction : Definition, Mechanism, Application, Explanation”