Table of Page Contents
What is Lossen Rearrangement?
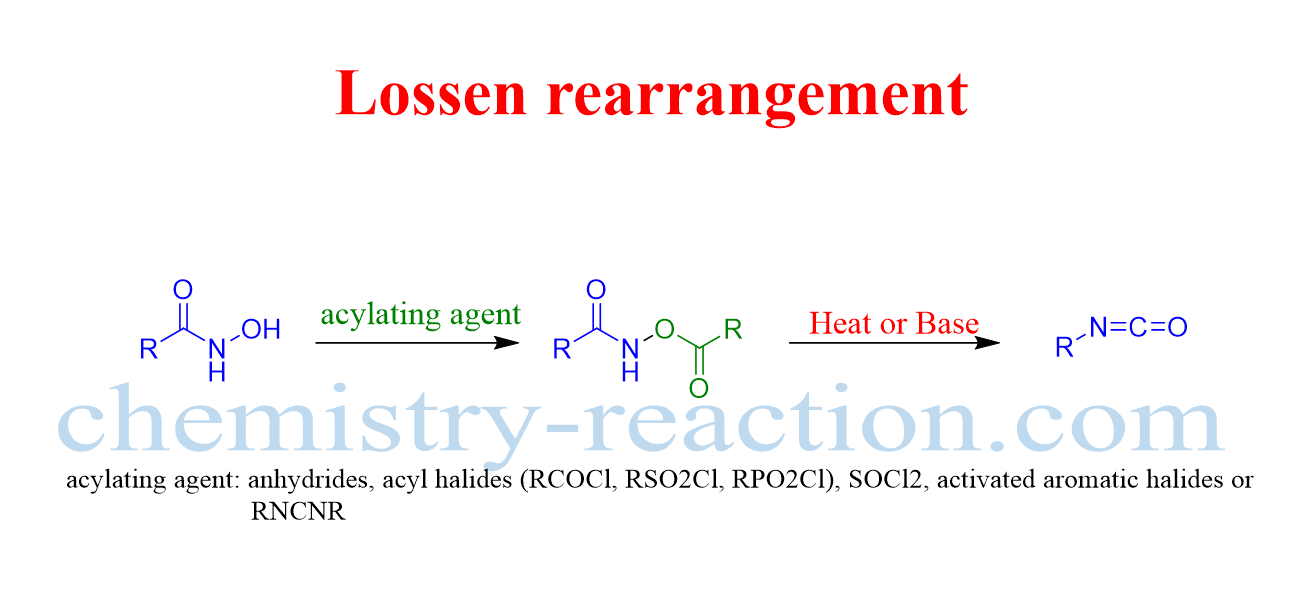
lossen rearrangement is a type of organic rearrengment reaction, where convertion of hydroxamate ester to an isocyanate take place via migration of alkyl or aryl group on the nitrogen atom of hydroxamate ester.
or
The conversion of O-acyl hydroxamic acids to the corresponding isocyanates is known as the Lossen rearrangement.
Further ureas can be generate in the presence of amines from The isocyanate and amines can generate in the presence of H2O. the lossen rearrangement from free hydroxamic acids is a modification of of the reaction.

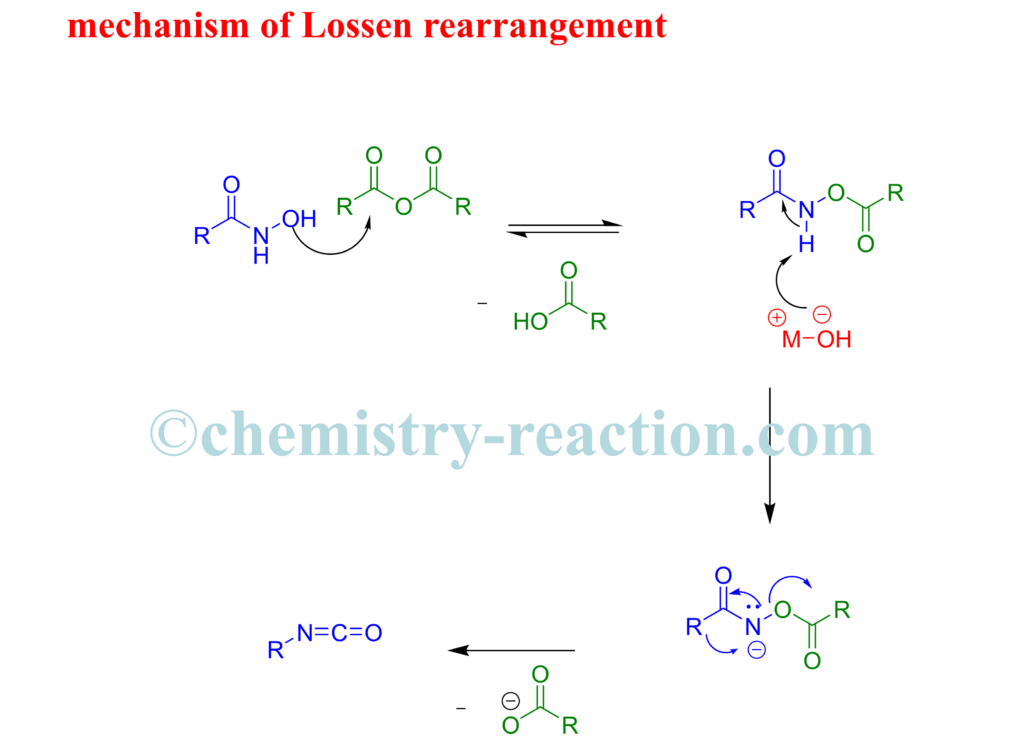
Mechanism of Lossen Rearrangement –
The Lossen rearrangement mechanism is closely similar to the Schmidt , Curtius-and Hofmann rearrangements.
The first step base will remove proton of the O-acyl hydroxamate at the nitrogen atom and the corresponding alkali salt will generate, which is unstable and instantly undergoes a rearrangement to the isocyanate.
The rate of the rearrangement : the more electron-withdrawing R is and the more electron-donating R then the rate will be higher.
See also : click chemistry
Related rearrangement reaction-
- Hofmann rearrangement
- Curtius rearrangement
- Beckmann rearrangement
- Sommelet-Hauser Rearrangement
- Smiles Rearrangement
References-
- Ritter Reaction
- Acetoacetic-Ester Condensation (Claisen Condensation)
- Favorskii Rearrangement
- Baeyer-Villiger Rearrangement (Baeyer-Villiger Oxidation):
- CSIR-UGC NET-JRF CHEMICAL SCIENCES (Chemistry) Syllabus 2022
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
2 thoughts on “Lossen Rearrangement Mechanism”