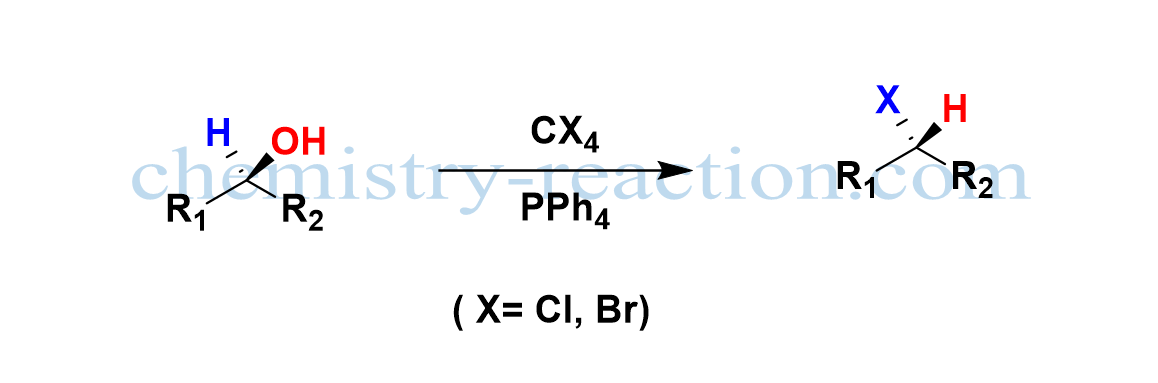Table of Page Contents
What is Appel reaction explain?
The Appel reaction is a common organic nucleophilic substitution reaction (SN2) employed to transform alcohol to a corresponding alkyl halide using the reagents tetrahalomethane (CX4) and triphenylphosphine (PPh4) with inversion of stereochemistry and high yield.
Mitsnobu Reaction mechanism is a closely related reaction to an Appel reaction where the inversion of stereochemistry takes the place of alcohol. the Appel reaction solvents are nonpolar, aprotic like DCM, Toluene, and tetrahalomethane.
Appel Reaction Mechanism:
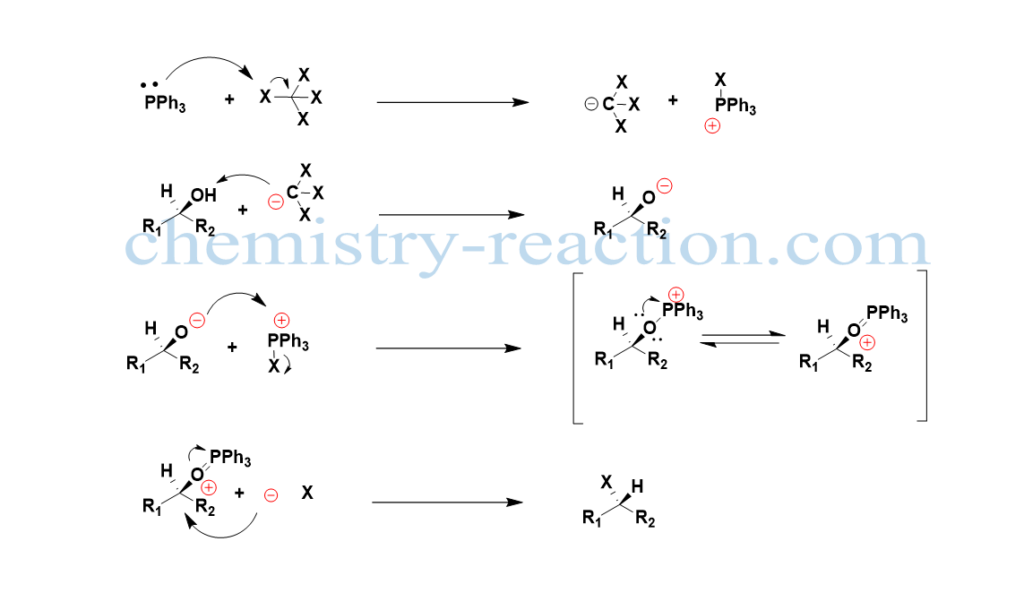
the mechanism of reaction proceeds by nucleophilic attack of PPh4 on C-X bod of tetrahalomethane (CX4) to generate phosphonium cation, the deprotonation of the alcohol produces the alkoxide ion. Then next, the nucleophilic alkoxide will attack phosphonium halide and become the oxyphosphonium intermediate by leaving the X– here, the oxygen is becoming the leaving group because of the strong P=O double bond. At last nucleophilic displacement will take place by liberated X– continuing with inversion of the configuration of asymmetric carbon. The by-product of the reaction is Triphenylphosphine oxide.
Related Reaction:
Reference:
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.