The Alder Ene reaction, also known as the Alder-ene reaction or the ene reaction, is a type of pericyclic reaction in organic chemistry. It involves the addition of a π-bond (ene) to an unsaturated double bond (eneophile) in the presence of a Lewis acid or a transition metal catalyst. The reaction proceeds through a concerted mechanism, meaning that both the bond-making and bond-breaking steps occur simultaneously.
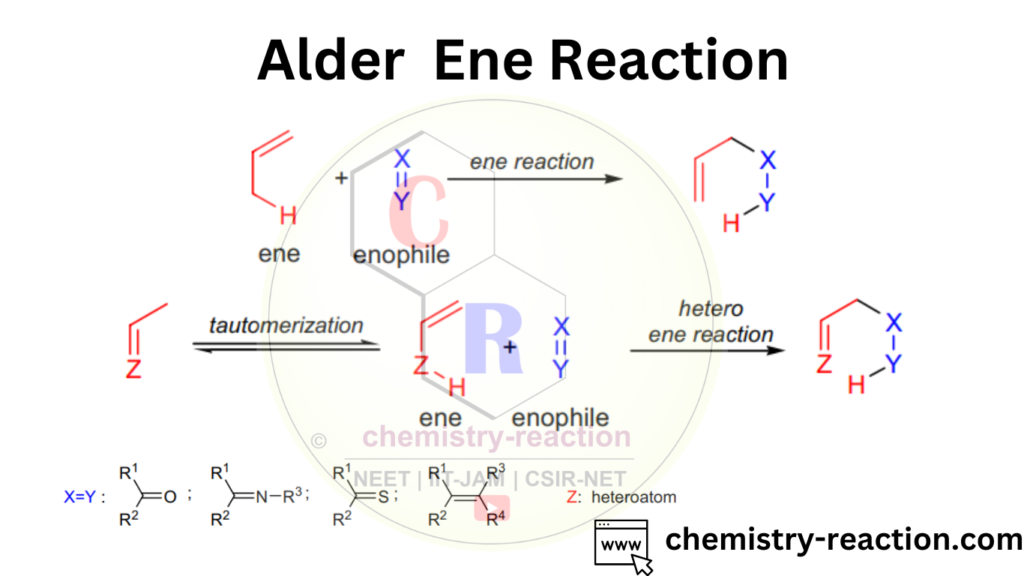
Table of Page Contents
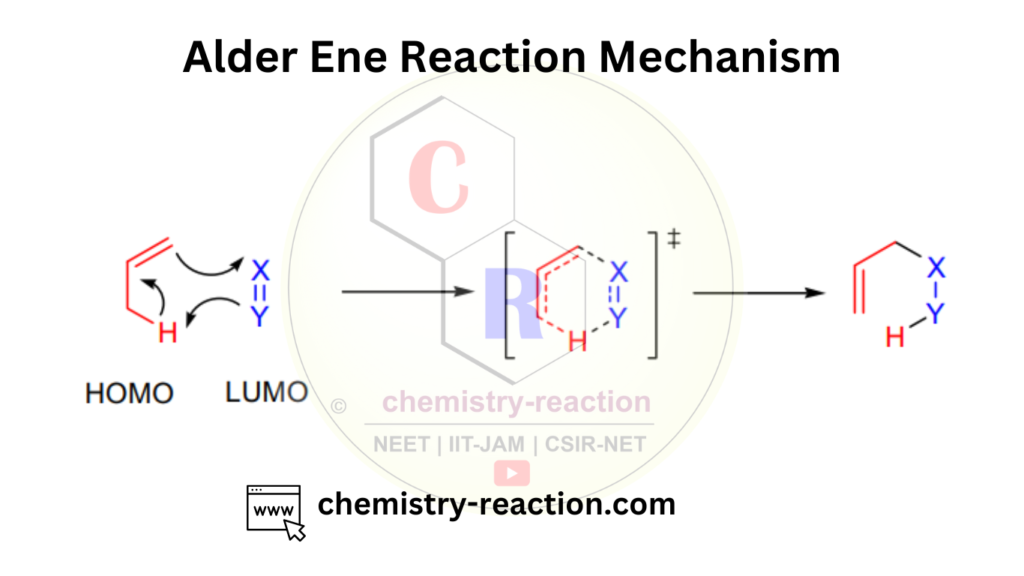
Alder-Ene Reaction Mechanism:
The Alder Ene reaction proceeds through a concerted pericyclic mechanism. The reaction involves the addition of a π-bond (ene) to an unsaturated double bond (eneophile) in the presence of a Lewis acid or a transition metal catalyst. Here’s a step-by-step explanation of the mechanism:
- Activation:
- Formation of the π-complex:
- Approach and transition state formation:
- Bond formation and bond breaking:
- Product formation:
This concerted mechanism allows for the efficient formation of new carbon-carbon bonds and the synthesis of complex molecular structures in a single reaction.
Ene Reaction Examples :
This is an examples of alder ene reaction
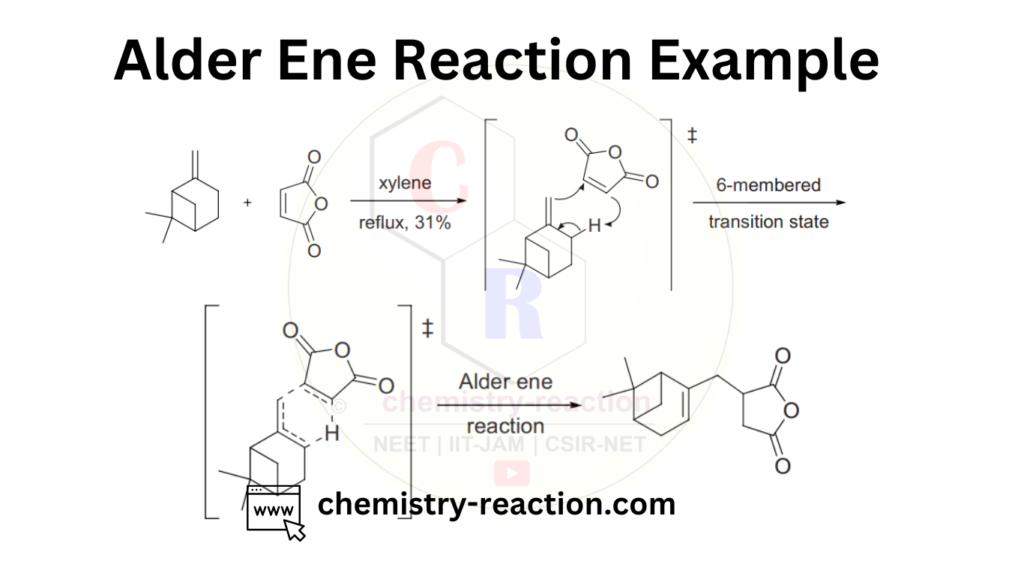
Intramolecular Ene Reactions :
This is an intramolecular alder ene reaction
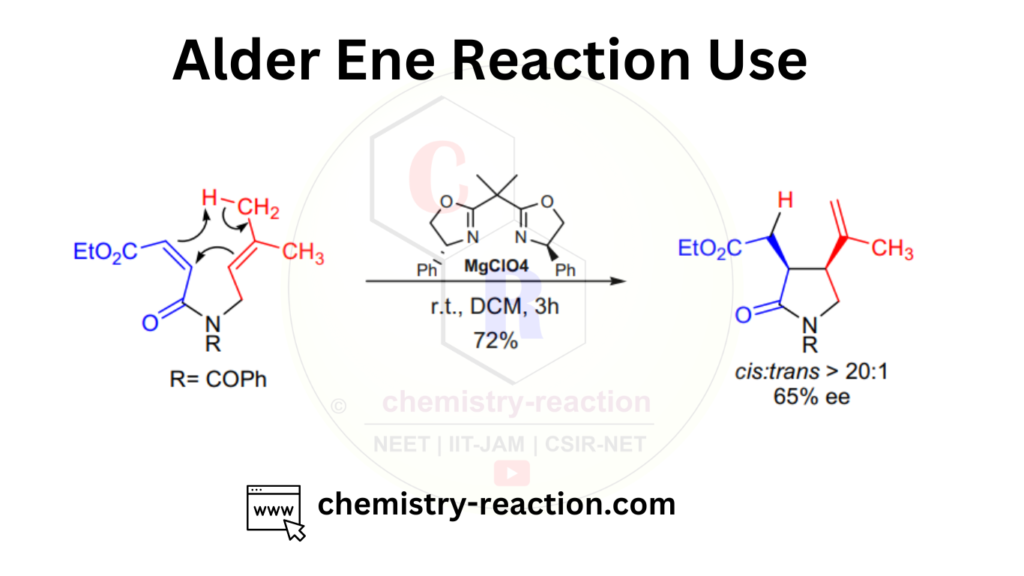
Ene Reaction in Pericyclic Reaction :
these are examples of ene reaction in pericyclic reactions
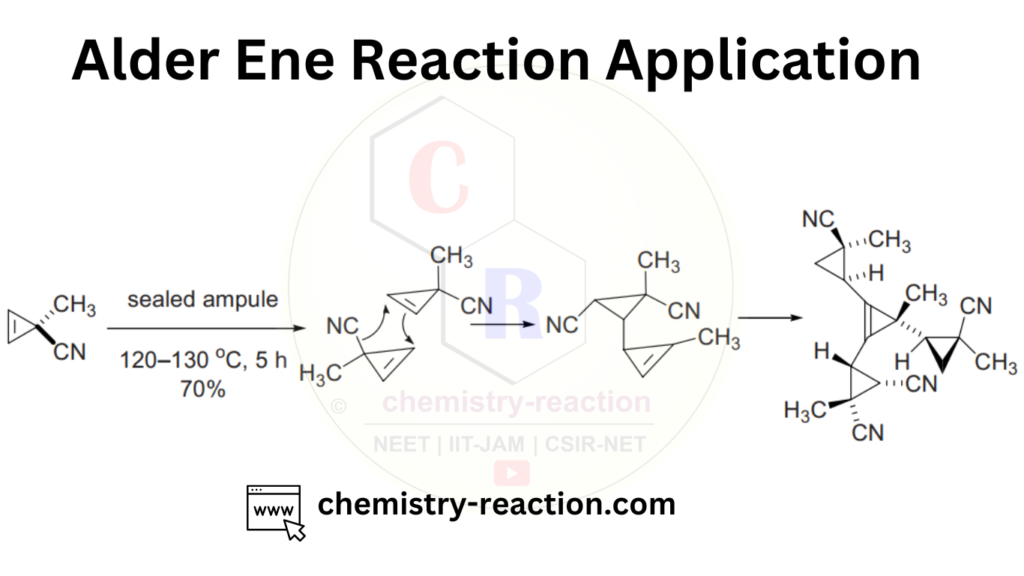
Related Reaction:
- Diels-Alder reaction
- Thiol-ene reaction
- Prins Reaction
References :
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
