Table of Page Contents
What is Prévost Reaction?
Prévost Reaction is two-step organic transformation, treatment of styrene with silver benzoate and iodine in dry benzene as solvent deliver the dibenzoate ester of the corresponding glycol. after hydrolysis leads the 1,2-diol (trans-1,2-diols), and it is referred to as the Prévost reaction.
Acyclic and cyclic alkenes are good substrates. Commonly used reagent is silver benzoate (R=Ph), silver carboxylates or thallium(I)acetate. When isolated and conjugated double bonds are both present in the same substrate, the usual dihydroxylation takes place on the isolated double bond prefer.

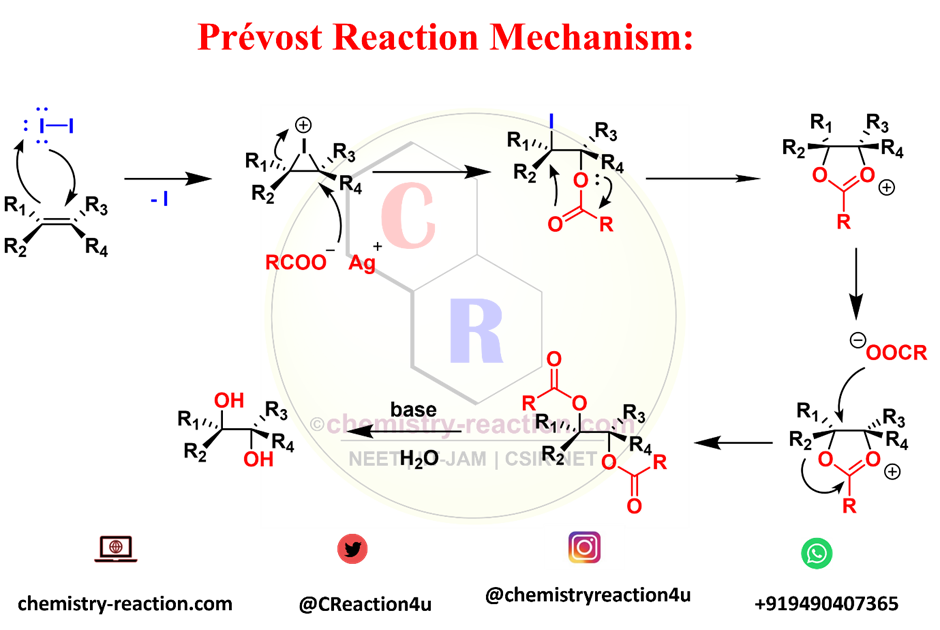
Prévost Reaction Mechanism:
The first step alkene reacts with iodine to form the cyclic iodonium ion.
Next, the iodonium ion is stereospecifically opened by the silver carboxylate to form the corresponding trans-1,2-iodo carboxylate.
The iodine is displaced intramolecularly by the carbonyl group of the carboxylate (anchimeric assistance) to form a cyclic cationic intermediate.
In the absence of water, this cation is opened with the inversion of configuration by the second equivalent of silver carboxylate to afford the trans-1,2-dicarboxylate which further convert in 1-2 diol.
Related reaction:
Reference:
- Charles Prévost (1933). “Sur un complexe iodo-argento-benzoïque et son application à l’oxydation des combinaisons éthyléniques en α-glycols”. Comptes rendus. 196: 1129.
- Briggs, L. H., Cain, B. F., Davis, B. R. Novel Prevost reaction. Tetrahedron Letters 1960, 9-11.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

