Hello, here are the Stevens rearrangement of quaternary ammonium salts and sulfonium salts with mechanism, examples and Stereochemistry all you need.
Table of Page Contents
Definition Stevens rearrangement :
Stevens rearrangement is a base-promoted [1,2]-migration rearrangement of quaternary ammonium salts or sulfonium salts to the corresponding tertiary amines or sulfides. Stevens rearrangement involves the [1,2]-migration of one of the groups on the nitrogen or sulfur atom.
Stereochemistry to Stevens rearrangement:
If the migrating group has a stereocenter, it will transfer with retention of configuration on migrating end, and it depends on substituents present in the migrating group. In the case of quaternary ammonium salts, retention in configuration at the migrating end occurs to a maximum extent than in the case of sulfonium salts.
The migratory aptitude of benzyl groups in the Stevens rearrangement: p-NO2>p-halogen>p-Me>p-OMe.
Stevens rearrangement of quaternary ammonium salts:
another key point in Stevens rearrangement, readily available quaternary ammonium salts are converted into tertiary amines using bases like NaH, KH, R-Li, Ar-Li, RO-Na, and RO-K depending on the acidity of the adjacent C-H bond. as a matter of fact an electron-withdrawing group like -Ar, -heteroaryl, -COR, -COOR, or -CN is required to stabilize carbanions at the beta position of both quaternary ammonium salts and sulfonium salts.

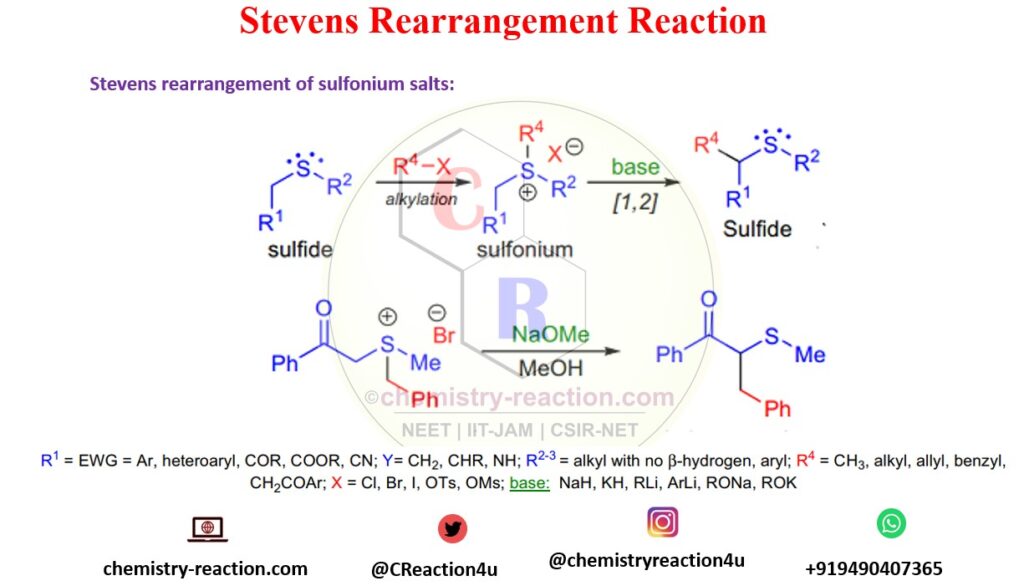
Stevens rearrangement of sulfonium salts:
sulfonium salts are shows transformation of sulfonium to sulfide in presence of base as shown in image of Stevens rearrangement.
Competing reactions for Stevens Rearrangement:
Competing reactions Sommelet-Hauser rearrangement can take place if one of the substituents are aryl or heteroaryl group. And also, Hofmann elimination may compete with the other two groups of ammonium salts that cannot contain hydrogen at their β-position.
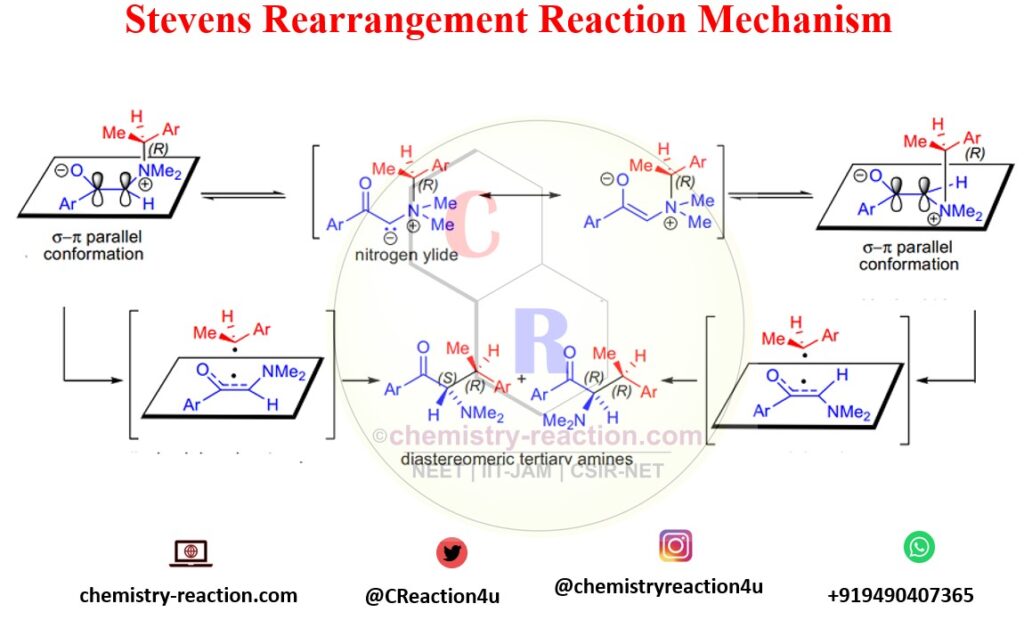
Reaction Mechanism Stevens Rearrangement :
The reaction mechanism of the Stevens rearrangement is not proved up to mark and has unjustified in organic chemistry. Firstly the Stevens Rearrangement reaction mechanism is proposed by T.S. Stevens.
Accordingly Stevens Rearrangement Mechanism, the first and essential step is the strong base will deprotonate substituent bearing electron-withdrawing properties of the ammonium salt and generate nitrogen ylide. Several reactions can occur in this condition. If considered the Stevens rearrangement is a concerted process, according to Woodward-Hoffmann rules, it is a symmetry-forbidden process.
Applications of:
Related Reaction:
- Sommelet-Hauser rearrangement
- Hofmann elimination
- Meisenheimer rearrangement
- Aza-Wittig rearrangement
- Aza-[2,3]-Wittig rearrangement
References :
- https://www.cambridge.org/core/books/abs/name-reactions-in-organic-synthesis/stevens-rearrangement/A407F59A4A2D5FDCEB2AB0520BF8C9FC
- https://www.sciencedirect.com/topics/chemistry/stevens-rearrangement
- https://en.wikipedia.org/wiki/Stevens_rearrangement
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “The Stevens Rearrangement:”