Stetter Reaction is a synthesis of 1,4-diketones and γ-oxo nitriles from aliphatic or aromatic aldehydes and a variety of activated α,β-unsaturated carbonyl compounds, β-unsaturated nitriles in the presence of a nucleophilic catalyst (catalytic amounts of sodium cyanide).
Table of Page Contents
What does Stetter Reaction explain?
The addition of aliphatic or aromatic aldehydes across activated double bonds in the presence of a nucleophilic catalyst for the synthesis of 1,4-diketones and γ-oxo nitriles is known as the Stetter reaction.
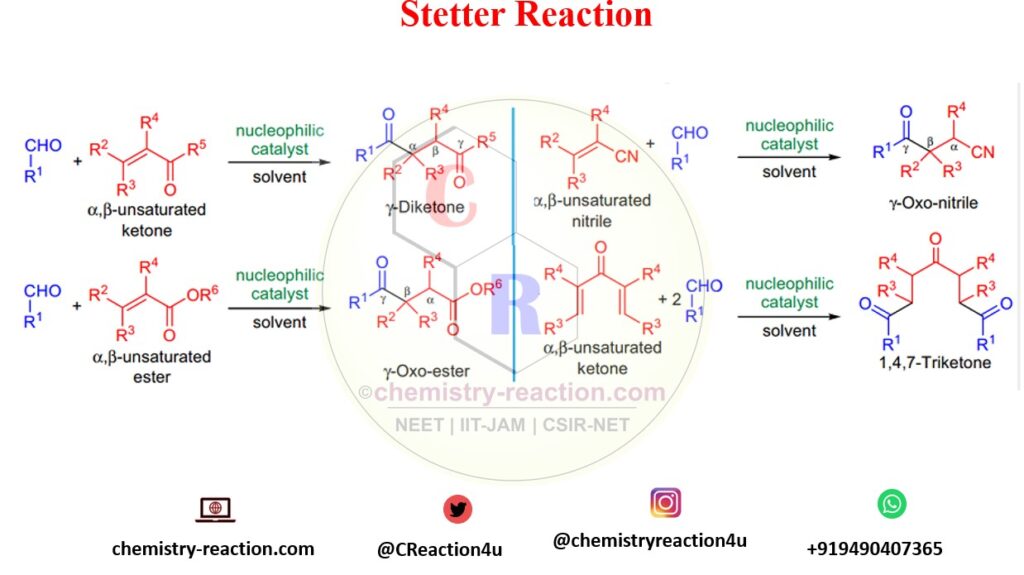
Many activated alkene substrates can be used, and the yields are incredibly high with α,β-unsaturated ketones. Straight-chain aldehydes tend to give higher yields than α-branched aldehydes
The limitation of the Stetter Reaction is that the reaction fails with aromatic aldehydes that have nitro substituents and 2,6-disubstituted aromatic aldehydes because of steric hindrance.
Stetter Reaction Mechanism
Mechanism of Stetter Reaction involve 3 steps as shown bellow :
- Reversible benzoin condensation
- Irreversible addition to the Michael acceptor
- Formation of catalytically active species from thiazolium salts
Asymmetric intramolecular or intermolecular Stetter reaction mechanism is an example of umpolung chemistry because aldehyde is converted from an electrophile to a nucleophile under standard reaction conditions.
Asymmetric Stetter Reaction mechanism involves the rapid, reversible formation of benzoins from aromatic aldehyde substrates; benzoins can be used instead of the aldehydes.

Applications of Stetter Reaction:
- The short synthesis of (±)-trans-sabinene hydrate, an important flavor chemical found in a variety of essential oils
from mint and herbs, was developed by C.C. Galopin - Synthesis of rac-hirsutic acid C has done by Trost and coworkers by employing a Stetter reaction.
Stetter Reaction Related Reaction :
- Benzoin condensation
- Baylis–Hillman reaction
- Paal-Knorr synthesis
References:
- https://www.organic-chemistry.org/namedreactions/stetter-reaction.shtm
- https://www.sciencedirect.com/topics/chemistry/stetter-synthesis
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejoc.200800506
- https://pubs.acs.org/doi/10.1021/ja904375q
- https://onlinelibrary.wiley.com/doi/10.1002/ajoc.202000378
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.


1 thought on “Stetter Reaction: synthesis of 1 4-diketones and γ-oxo nitriles”