Vilsmeier–Haack formylation reaction : Mechanism | Applications
The formylation of electron-rich aromatic compounds with a mixture of dimethylformamide and phosphorus oxychloride POCl3 is commonly referred to as the Vilsmeier reaction, or sometimes the Vilsmeier-Haack reaction, from the names of the two German chemists who first described it in 1927.
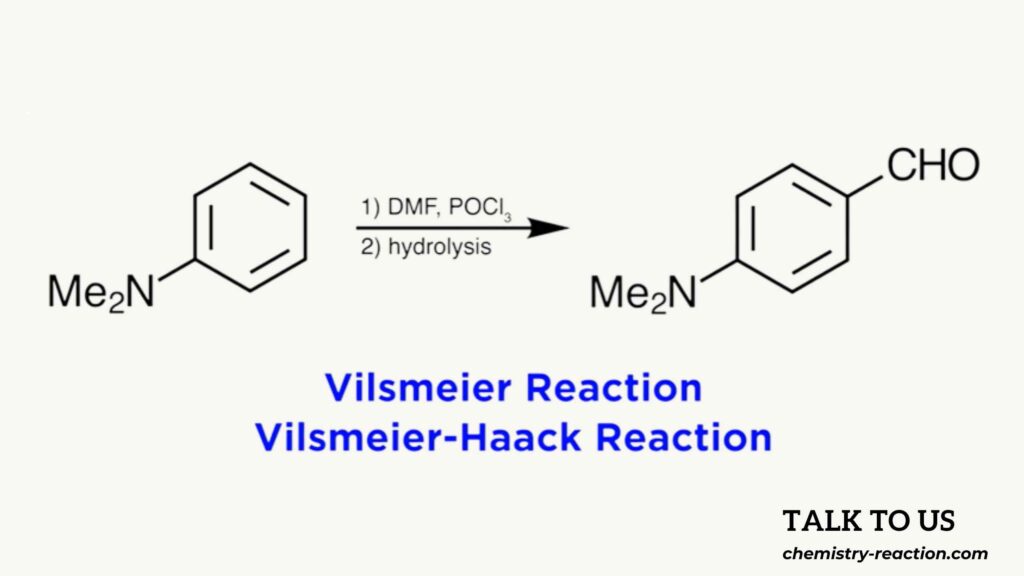
A formylation is a reaction in which a formyl group is introduced to a compound, meaning a CHO group, resulting in an aldehyde. Other reagents can also be used in place of DMF, and other activators can be used as well, like for example phosgene or phosphorus pentachloride, but liquid phosphorus oxychloride is convenient to use and is the preferred reagent.
Table of Page Contents
Mechanism of Vilsmeier-Haack Reaction :
How do you make Vilsmeier-Haack reagent?
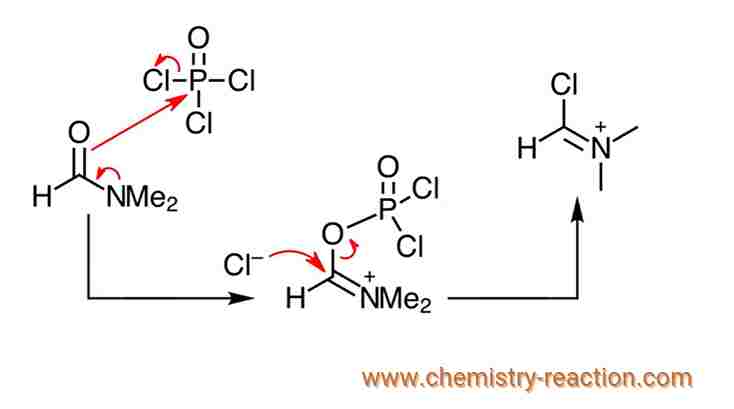
In the first part of vilsmeier haack reaction mechanism, the Vilsmeier reagent is formed. As we can see, nitrogen pushes its lone pair onto carbon, then oxygen attacks phosphorus, and one of these chlorines will leave. Because of this we now have chloride in solution, which will attack this carbon, and this carbon-oxygen bond will cleave. This is the Vilsmeier reagent.
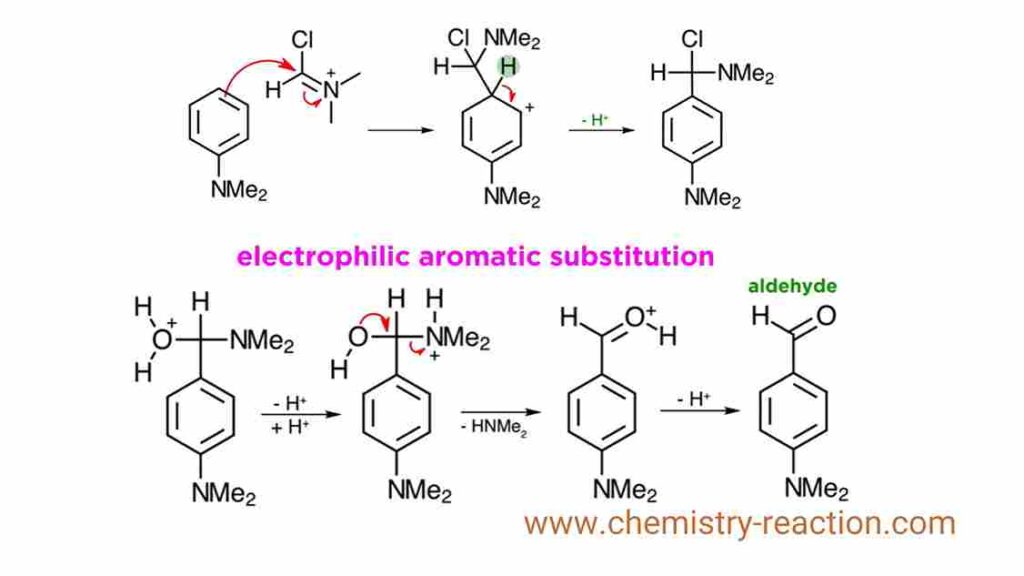
Once formed, the reaction with the aromatic substrate takes place. Electrophilic aromatic substitution takes place at this electrophilic carbon, neutralizing the nitrogen, and yielding this intermediate. Loss of a proton finishes up the EAS reaction. Then nitrogen uses its lone pair to kick off chlorine, which leads to the iminium ion. This can be isolated in some cases, especially if it crystallizes. However, such intermediates are not stable to work up and they will readily hydrolyze. This should be familiar, but just to be thorough, water attacks and neutralizes the nitrogen, proton transfers occur, then a lone pair on oxygen kicks off the amine, one more proton transfer, and we are left with the aldehyde, which is the product of the reaction. vilsmeier complex
what is Vilsmeier reagent?
The Vilsmeier reagent is a powerful electrophile, and one reason this reaction is still synthetically important nearly a century after its development is that we are still exploring the range of substrates that react with it, thus broadening the scope of this reaction.
Vilsmeier-Haack Reaction Application
Formylation of alkene
Simple application of vilsmeier reaction, alkenes, such as these shown here, can react with a Vilsmeier reagent, which we will understand to be present once these two reagents interact, as in the first step in the mechanism we just went over. In most cases, the reaction leads to formylation at the less substituted carbon, although these reactions are not always high yielding, while also producing a mixture of E and Z isomers.

Formylation of acid
An interesting extension applies to carbonyl compounds, which can react at the active methylene moieties via their enol forms. And finally, after mild hydrolysis, the β-chloro-α,β-unsaturated aldehyde is achieved as a mixture of E and Z geometric isomers.
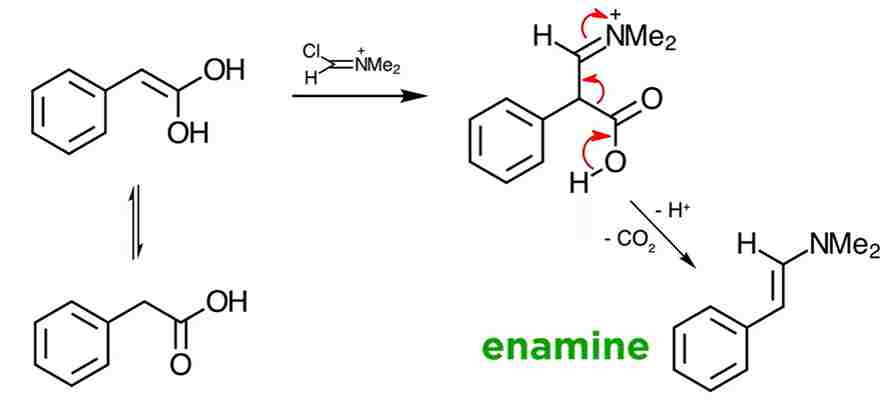
Finally, we can examine the situation involving carboxylic acids. Since carboxylates have an α-methylene group, a double formylation with accompanying decarboxylation is observed. Perhaps one day you can think of a new application yourself!
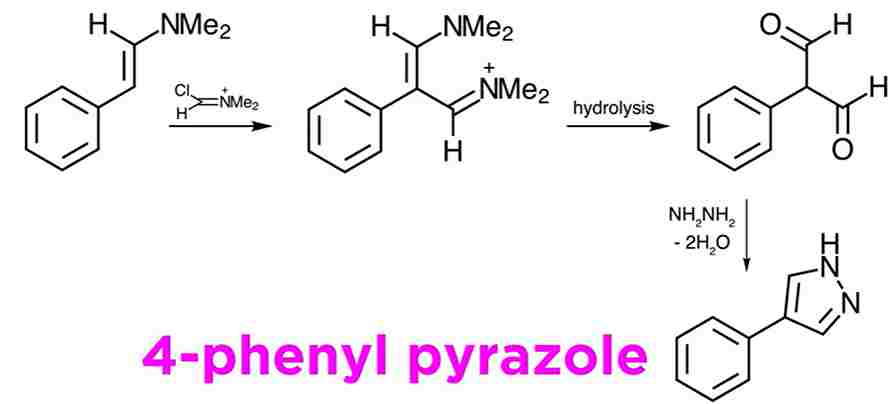
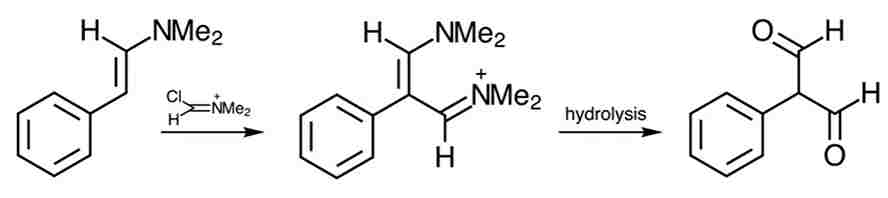
Related reaction:
- Reimer-Tiemann reaction
- Kolbe-Schmitt reaction
- Gatterman formylation
- Gatterman-Koch formylation
- Houben-Hoesch reaction
References:
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
1 thought on “Vilsmeier–Haack formylation”