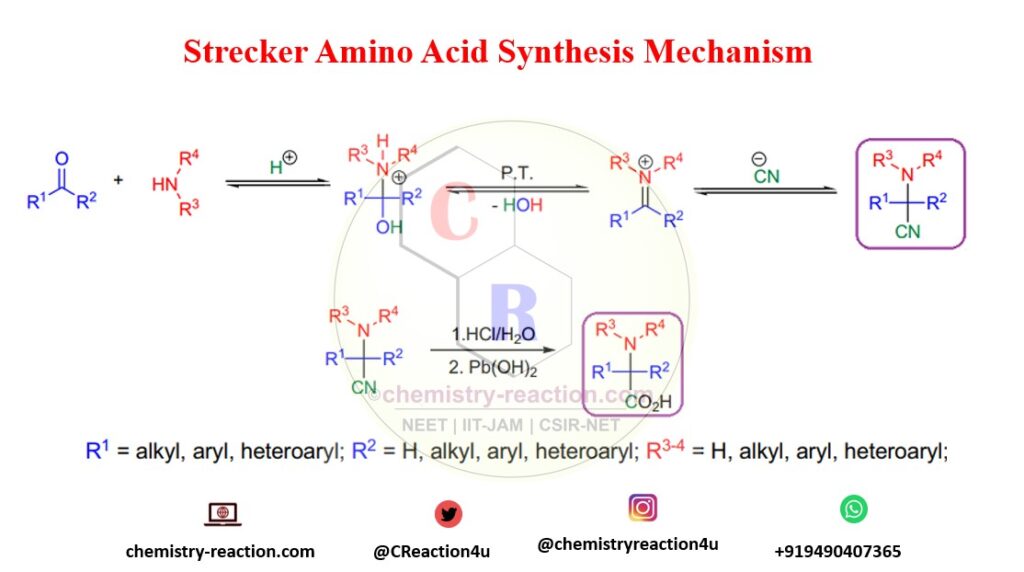
The condensation reaction of ketone or aldehyde with an ammonia or primary amine and various alkali cyanides (e.g., hydrogen cyanide, KCN, Na-CN) to afford the corresponding α-amino nitrile is known as the Strecker reaction.
The most well-known use of the Strecker reaction, α-amino nitriles, is their hydrolysis under acidic or basic conditions to obtain α-amino acids (Strecker amino acid synthesis).
Table of Page Contents
Features of Strecker Synthesis of Amino Acids:
- Strecker multicomponent reactions were also known as Strecker synthesis alanine.
- The Strecker synthesis reaction is a one-pot three-component coupling reaction called Strecker multicomponent reaction. Aldehydes and ketones will give a good yield. In preparation of amino acid by Strecker synthesis, ammonia, primary, or secondary amine can be used as amine substrate.
- The HCN is exceptionally toxic, so various alkali cyanides are usually used (e.g., KCN, NaCN) in buffered aqueous media.
- In Strecker amino acid synthesis, adding HCN to preformed aldimines and Ketimines (even iminium salts) or oximes and hydrazones leads to N-substituted α-amino nitriles. It is a concern for Strecker synthesis in green chemistry.
- In Strecker reaction conditions, hydrolysis of α-amino nitriles gives α-amino acids, reduction with metal hydrides affords 1,2-diamines, while strong bases can deprotonate at the α-carbon (if R2 =H), and the resulting carbanion can be trapped with a variety of electrophiles (umpolung)

Asymmetrical Strecker reactions can be conducted while optically active amines generate chiral imines, which give rise to enantio-enriched α-amino nitriles upon the addition of cyanide ions and using organ-catalysts or chiral metal catalysts.
In Strecker asymmetric reaction, optically active amines generate chiral imines in the catalytic asymmetric Strecker reaction, which gives rise to enantio-enriched α-amino nitriles upon the addition of cyanide ions.
Strecker Synthesis Mechanism:
Synthetic Applications:
The asymmetric Strecker reaction was used to construct the key tetramethyltryptophan subunit.
Related Reaction of Strecker Synthesis:
- Essential Amino Acids List
- Asymmetric Strecker reaction
- Schmidt reaction
Reafferences :
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
