The reaction of aza-ylides ( also called as iminophosphoranes) with different carbonyl molecules is called the
aza-Wittig reaction. In this post described the Aza-Wittig Reaction Mechanism with Examples.
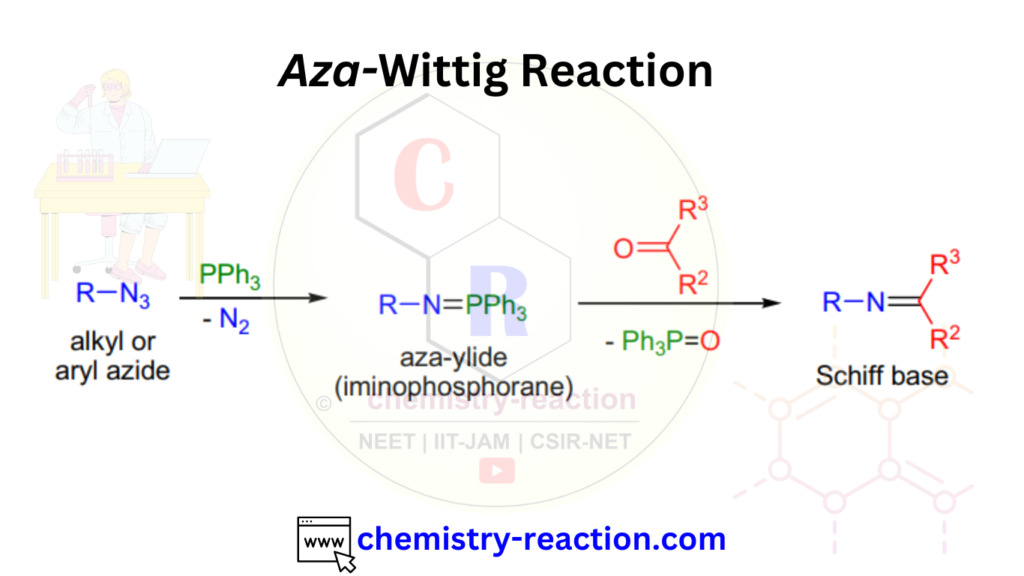
Table of Page Contents
Aza-Wittig Reaction Mechanism
Mechanism of Aza-Wittig Reaction are defined in two steps as follow
First step : the PPh3 reacts with an alkyl-azide and generate an iminophosphorane by loosing of nitrogen N2 (also called Staudinger reaction).
Second step: the nucleophilic nitrogen of the iminophosphorane attacks the carbonyl group and four-membered intermediate (oxazaphosphetane) generate, from oxazaphosphetane product Schiff base and the by-product triphenylphosphine oxide are released. this is the Aza-Wittig Reaction Mechanism

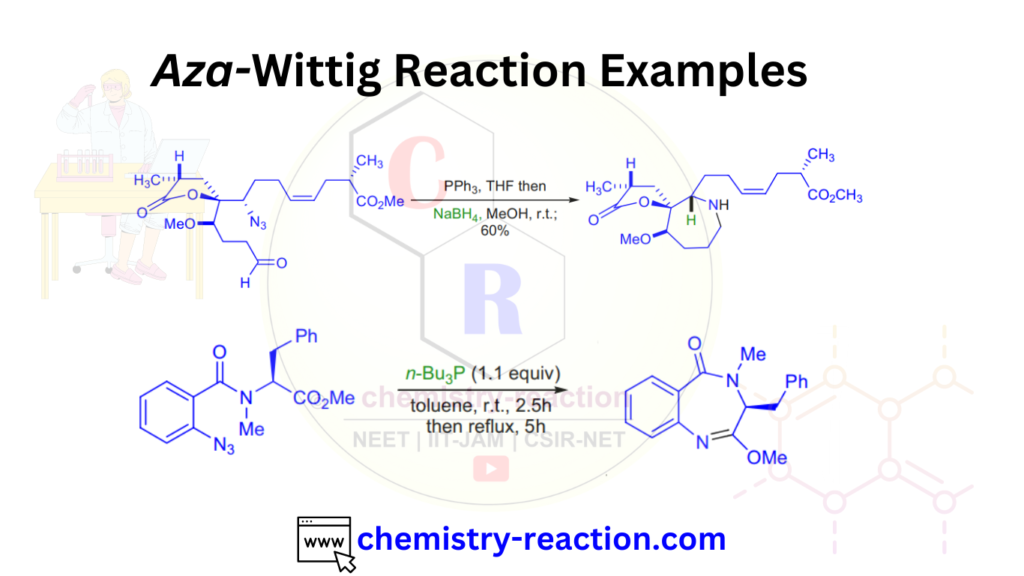
Aza-Wittig Reaction Examples:
Aza-Wittig Reaction with Mechanism and Examples has been shown below
Related Reactions:
- Wittig Reaction
- Staudinger Reaction
References:
- Catalytic Wittig and aza-Wittig reactions ^
- Strategic Applications of Named Reactions in Organic Synthesis ^^
- Aza-Wittig Reaction: Mechanism | Examples
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

2 thoughts on “Aza-Wittig Reaction: Mechanism | Examples”