Ketene is a valuable organic reagent, also known as ethenone, and it is high reactivity. German chemist Hermann Staudinger revealed the ketene preparation of organic compounds end of the 19 century. According to the Merck Index, ketene gas has a “penetrating” odor, colorless and toxic. Ketenes have sp character carbon atoms bonded with the heteroatom and are exceedingly electrophilic. ketene formula is C2H2O. these are the general features of ketene.
Ketene can be produced with different heteroatoms bonded to the sp carbon atom, such as O, S (thioketene) or Se (selenoketene) heteroatoms bonded to sp carbon ketenes can be prepared with different methods as described below. Ketenes are highly labile and challenging to store. Ketenes have played an essential role in synthesizing antibiotics amoxicillin and penicillin.
Table of Page Contents
Ketene a useful reagent: general features, structure
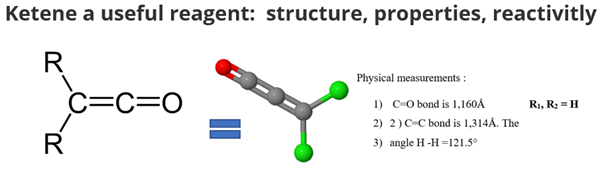
Physical aspect of ketene :
- C=O bond is 1,160Å
- C=C bond is 1,314Å. The
- angle H -H =121.5°
Alexander Hinz and Jose M. Goicoechea recently published a report for 4-membered arsenic heterocycles synthesis at Oxford University (UK). They used a three-step sequence that starts from elemental arsenic to prepared arsenic analog. Ketenes and carbodiimides readily react with 2-arsaethynolate (As=C=O–) to form 4-membered arsenic heterocycles.
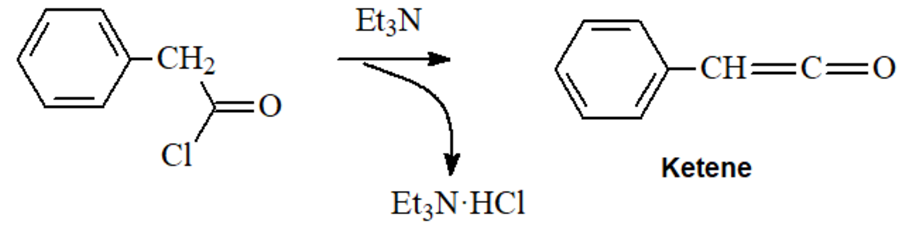
Most common methods for preparation of ketenes are:
- dehydrohalogenation of acid chlorides by trialkylamines
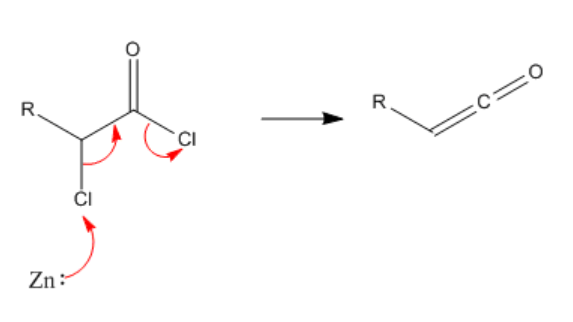
- dehalogenation of α-halo acid chlorides by zinc or zinc-copper alloy to form dihaloketenes.
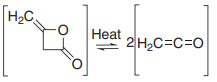
- thermal or photochemical opening of cyclobutenones.
- Wolff rearrangement of α-diazoketones.

- pyrolysis of anhydrides followed by bulb-to-bulb distillation.
- pyrolysis of esters
- cracking commercially available diketene at atmospheric pressure leads to ketene.
Reactivity and Application of Ketene :
Reactions and applications of ketene in organic synthesis: Formation of carboxylic acid esters, Formation of carboxylic acid amides, Hydrolysis of ketene

A) Reaction of ketenes with alkenes :
- The reaction of ketene with alkene gives to cyclobutanones as product.

- The order of reactivity with simple alkenes with ketene is trans olefin < cis olefin < cyclic olefin< linear diene < cyclic diene.
- Stereochemistry will retain around the double bond. And the polarization of the double bond decides regiochemistry.
- ketene itself is not reactive toward double bonds; usually dichloroketene is used instead, followed by dehalogenation by zinc-copper alloy catalyst.
- in case of perfluorinated ketenes and alkoxybutadienes, the reaction may lead to the [4+2] cycloadducts, and in addition to simple alkenes, allenes, enamines, and enol ethers also undergo the cycloaddition, although the yields are generally lower.
B) Reaction of ketenes with imines:
- the reaction is of particular importance because it leads to the formation of azetidinones (also called as β-lactams).
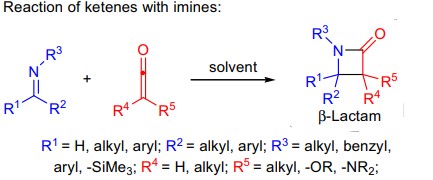
- the reaction is usually carried out thermally or photochemically using acid chloride and triethylamine or α-diazoketones as the ketene precursors.
- the diastereoselectivity of the resulting β-lactams is generally high.
- asymmetric versions were developed by employing chiral auxiliaries attached to the imine or the ketene.
- asymmetric catalytic methods utilizing chiral amine bases were also developed; and
- when the reaction is carried out in sulfur dioxide, it leads to the formation of 2,3-diphenylthiazolidin-4-one-1,1-dioxide derivatives.
- In addition to the above compounds, acetylenes, thiocarbonyls, isocyanates, carbodiimides, N-sulfinyl amines, nitroso- and azo compounds also undergo a formal [2+2] cycloaddition with ketenes.
C) Reaction of ketenes with aldehydes and ketones:
- the reaction leads to the formation of 2-oxetanones also called as β-lactones.
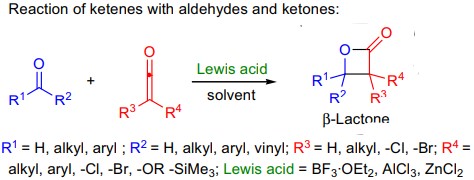
- these reactions usually require Lewis acid activation, and the most common Lewis acids are boron trifluoride etherate, aluminum chloride, and zinc chloride;
- amines can also be utilized as catalysts.
- carbonyls strongly bearing electron-withdrawing substituents do not require activation.
- a wide array of ketene substrates can be used, although aryl- and diarylketenes are generally unreactive; and
- asymmetric versions of the cycloaddition have been developed by utilizing chiral amine bases as catalysts.
Related reactions:
- Wolff rearrangement
- Johnson-Claisen- or ortho ester Claisen rearrangement
- Danheiser benzannulation
- alder ene reaction
- staudinger ketene cycloaddition
References:
- https://www.acs.org/content/acs/en/molecule-of-the-week/archive/k/ketene.html#:~:text=Ketene%20(systematic%20name%20ethenone)%20is,ketene%20in%20an%20argon%20matrix.
- https://www.sciencedirect.com/topics/chemistry/ketene
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

1 thought on “ketenes: General Feature, Preparation, Reactivity, Application”