Table of Page Contents
What is Claisen Condensation?
Formation of aceto-acetic-ester by the reaction of sodium ethoxide with ethyl acetate is called Claisen Condensation reaction or The Claisen Condensation reaction between Esters (containing α-hydrogens), in the presence of Bases such as sodium ethoxide, gives β-ketoesters via stabilized anion of the β-keto Ester.
If two different esters are used, an basically mixture of all four products is obtained, and the preparation does not have high synthetic value.
One of the Ester has enolizable α-hydrogens, and the other is non-enolizable (aromatic esters or carbonates). It’s called crossed Claisen. Yield will increase if a more substantial base, e.g., sodium amide or sodium hydride instead of metallic alkoxide bases.
Dieckmann Condensation is the intramolecular version of the Claisen condensation. The aldol condensation reaction is closely related to the Claisen condensation reaction. In the aldol condensation involves the addition of enolates to aldehydes or ketones and Claisen condensation involves the addition of enolates to ester.
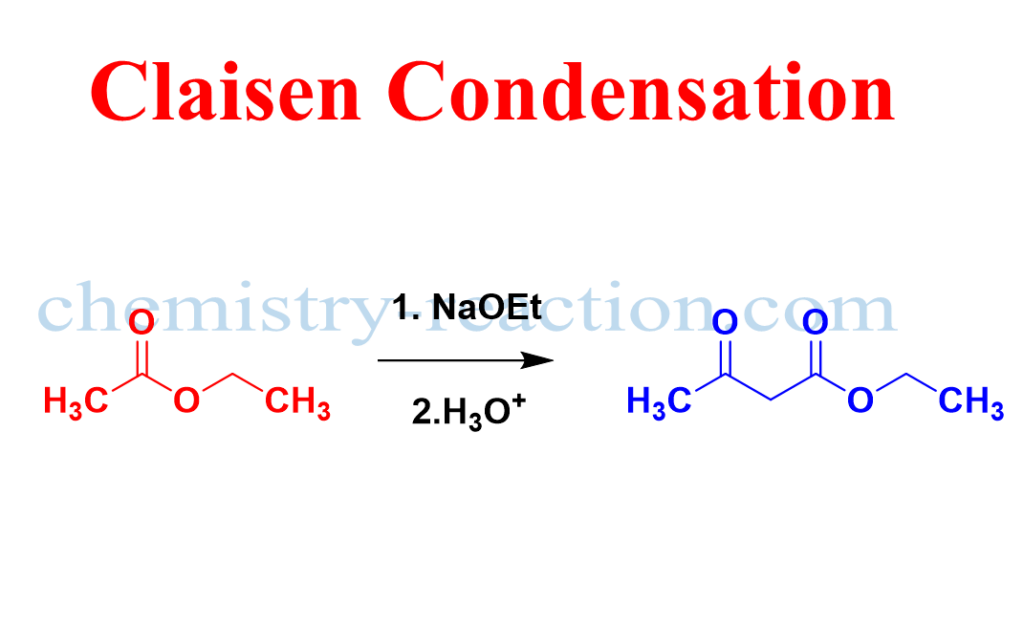
Mechanism of Claisen Condensation:
The mechanism for the synthesis (claisen condensation) of acetoacetic Ester from two esters. The step-by-step general reaction mechanism of the Claisen condensation reaction is given below.

Related Reaction:
References:
- Appel, R. Angew. Chem. Int. Ed. Engl. 1975, 14, 801–811.
- Smith, Janice Gorzynski. Organic Chemistry: Second Ed. 2008. pp 905–906
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.


4 thoughts on “Acetoacetic-Ester Condensation (Claisen Condensation)”