Schotten-Baumann reaction is a well-known name reaction in organic chemistry Synthesize of Amide and Ester. The synthesis of amides from amines and esters from alcohols with anhydrides or acyl halides in an aqueous inorganic or organic bases like NaOH.
The Schotten-Baumann reaction is a reaction of phenol with benzoyl chloride that gives benzoic acid phenolic esters. schotten-baumann reaction reagent are acyl halide or anhydride, organic base and or Lewis acid.
A facile method for synthesizing N-benzoyl piperidine from piperidine and benzoyl chloride in water and the presence of sodium hydroxide has been reported by Schotten. with the same condition, preparation of benzoic acid esters from alcohols and benzoyl chloride has established by Baumann.
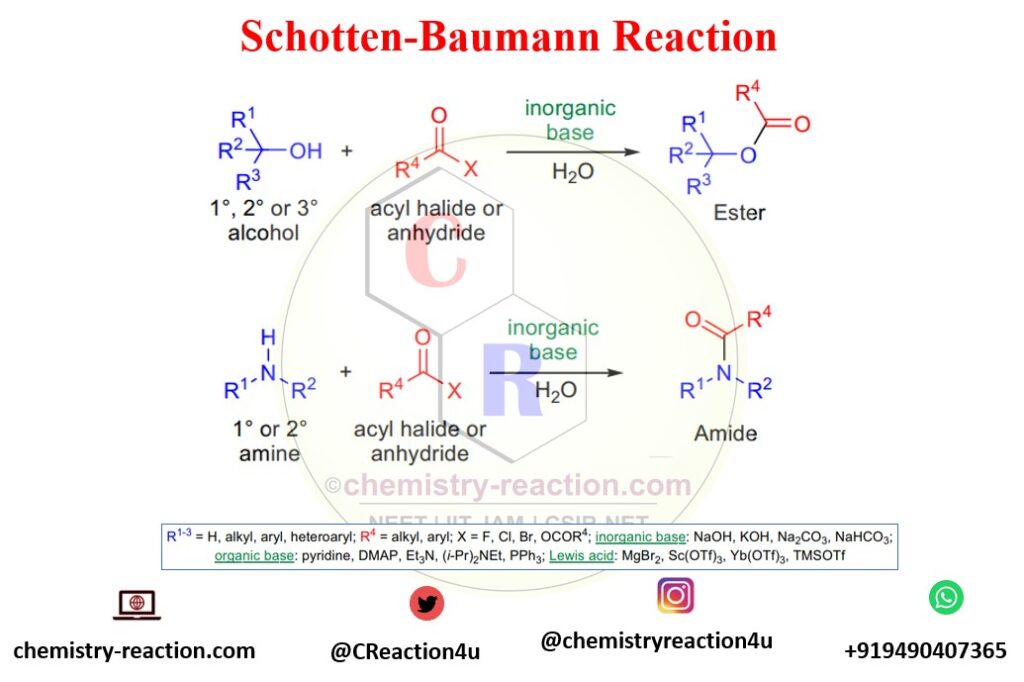
The reactivity order for alcohols Schotten-Baumann reaction for ester synthesis is 1°>2°>3°. The reaction is usually slow for sterically hindered secondary and tertiary alcohols.
Table of Page Contents
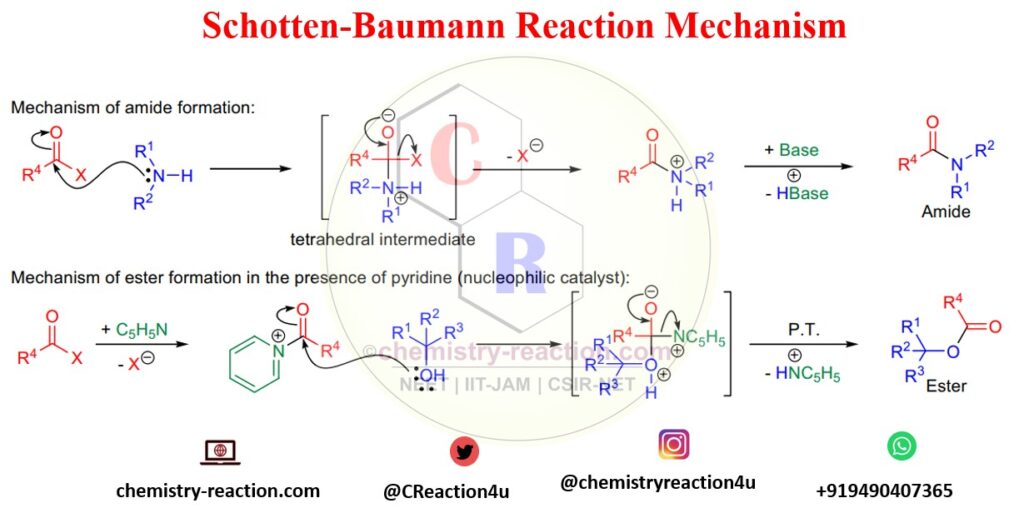
Schotten Baumann Reaction Mechanism :
1. Mechanism of Schotten Baumann for amide synthesis
In the first step of Schotten-Baumann’s reaction of an amine with benzoyl chloride, the nucleophile (amine) will attack carbonyl carbon and give a tetrahedral intermediate. Then the negative Oxygen anion will push leaving the group and providing amide in the presence of a base.
2. Mechanism of Schotten Baumann for ester formation:
In the benzoylation of phenol mechanism, first, the nucleophilic catalyst NC5H5 will attack the carbonyl carbon of benzoyl chloride and generate a cationic intermediate, as shown in the below image.
Next step, phenol or alcohol hydroxy OH will attack on the cationic intermediate and gives ester by leaving HN+C5H5.
Use of Schotten Baumann Reaction in synthesis:
Some of the applications of Schotten-Baumann reaction inorganic synthesis exist, shown below.
- Preparation of peptides by Fischer’s synthesis.
- Preparation of benzamide from phenethylamine and benzoyl chloride
- synthesis of N-vanillyl Nonanamide or capsaicin.
- It is used in benzylamine synthesis by acylation.
- Preparation of peptides by Fischer’s synthesis.
Related Reaction:
- Mitsunobu reaction
- Williamson ether synthesis
- Stobbe condensation
- Tishchenko reaction
- Polonovski reaction
- Ritter reaction
References :
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cber.19030360356
- https://en.wikipedia.org/Baumann_reaction
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cber.188401702178
- Total Synthesis of the Fumiquinazoline Alkaloids: J. Org. Chem. 2000, 65, 4, 1022–1030
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.