Sakurai allylation reaction is one of the most vital carbon-carbon bond-forming reactions in organic chemistry used to synthesize homoallylic alcohols. Sakurai reported the preparation of homoallylic alcohols from the reaction of aldehydes or ketones with allylsilanes in the presence of stoichiometric quantities of TiCl4. The Sakurai reaction is also known as Hosomi-Sakurai Reaction.
In this reaction, primarily electrophiles are aldehydes, acetals, ketones, and ketals for synthesis of homoallylic alcohols.
Lewis acids like AlCl3, BF3·OEt2, SnCl4, and EtAlCl2 can be used along with Ti-Cl4. This reaction is highly regioselective, the electrophile attacking at the C3 position of the allylsilane. C1 substituted allylsilanes give the (E)-alkene product. instead of TiCl4.
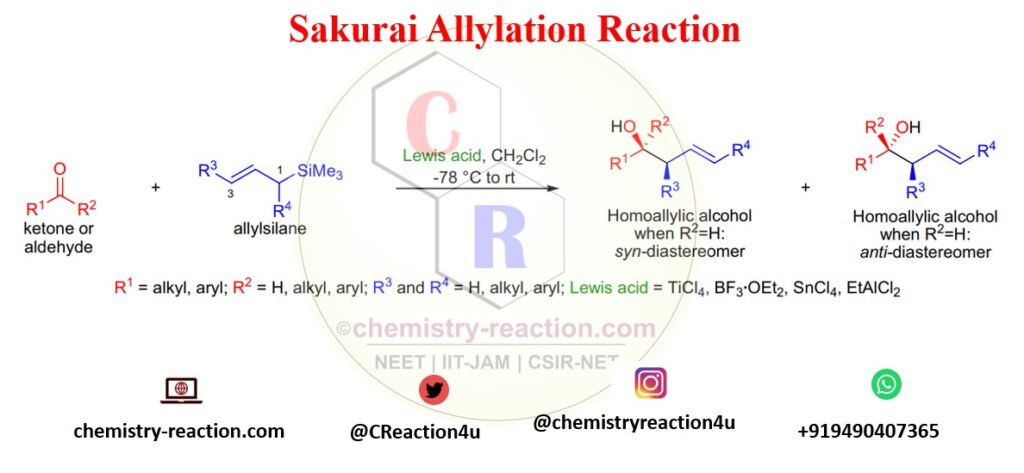
Table of Page Contents
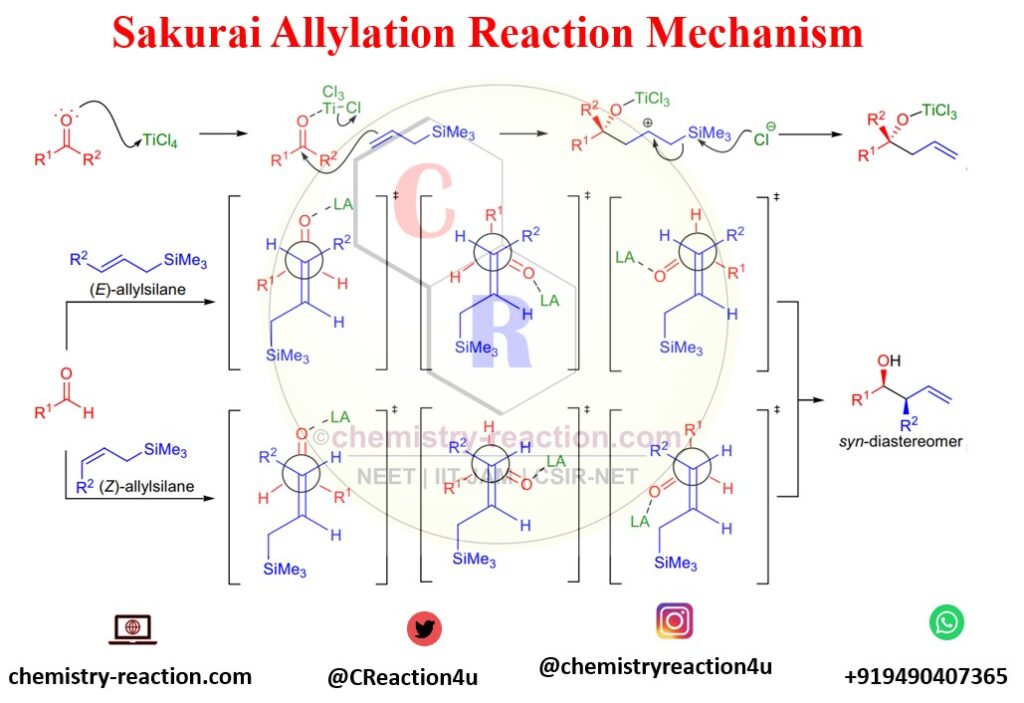
Mechanism of Sakurai Reaction (Allylation):
Lewis acid activates the carbonyl group in the first step of the Sakurai Reaction (Allylation). Subsequently, alkene will attack carbonyl carbon and generate silyl-stabilized Carbocation. The next step is homoallylic alcohol synthesis via losing trimethylsilyl by attacking Cl– anion.
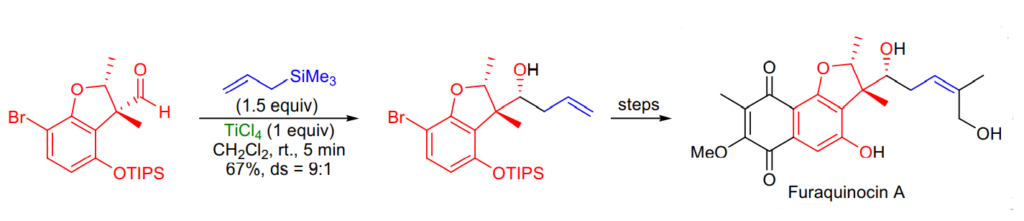
Applications of Sakurai Reaction:
Related Reaction:
- Claisen Condensation
- Grignard Reaction
- Tsuji-Trost Reaction or Trost Allylation
References :
- https://pubs.acs.org/doi/abs/10.1021/jo01258a052
- https://www.sciencedirect.com/science/article/abs/pii/S0040403900780440?via%3Dihub
- https://www.organic-chemistry.org/namedreactions/hosomi-sakurai-reaction.shtm
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
