The Saegusa oxidation is known for the regioselective introduction of the α,β carbon-carbon double bond to cyclic and acyclic ketones via the Pd-mediated oxidation of the corresponding silyl enol ether. its also known as Saegusa–Ito oxidation.
T. Saegusa reported oxidation of silyl enol ethers in the presence of stoichiometric amounts of Pd(OAc)2 and p-benzoquinone in acetonitrile at room temperature to afford the corresponding α,β-unsaturated carbonyl compounds in 1978.
Stereochemistry: predominantly (E) stereoisomer will form in this oxidation even if substrate silyl enol ether was a mixture of (E) and (Z) stereoisomers.
Both acyclic and cyclic silyl enol ethers undergo the Saegusa Oxidation transformation . Drawback of the this oxidation is high cost of the palladium acetate.
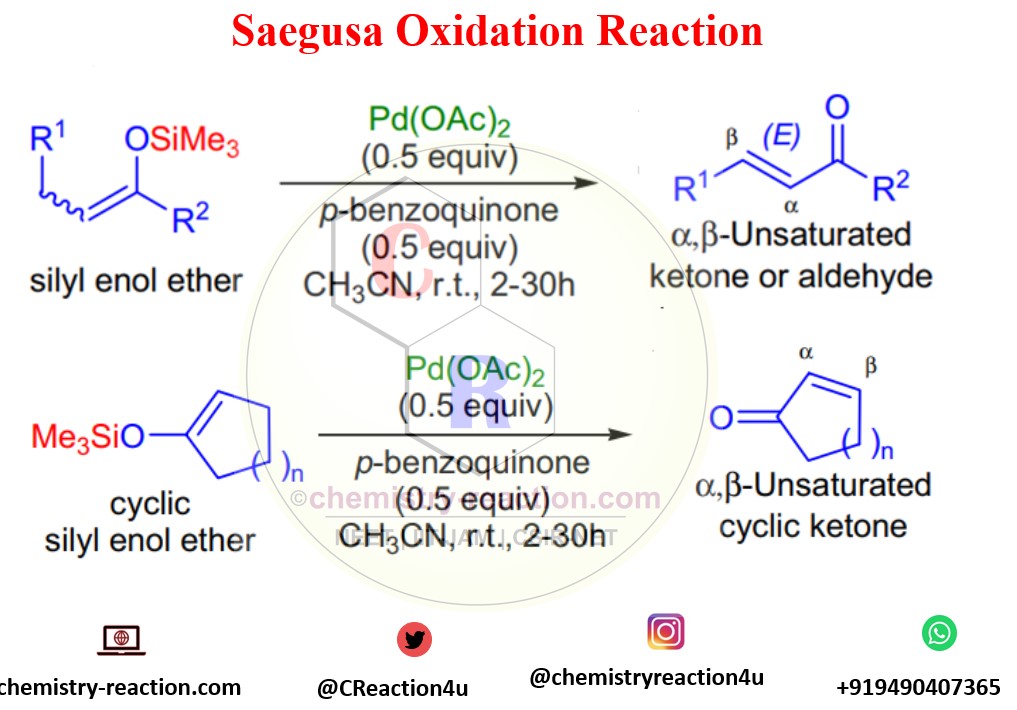
Table of Page Contents
Modifications of Saegusa–Ito Oxidation :
- Larock modifications of Saegusa–Ito oxidation = 10 mol% of Pd(OAc)2 instead of stoichiometric amount and oxygen atmosphere in DMSO.
- Nicolaou oxidation : use of IBX and IBX-N-oxides instead of Saegusa Oxidation condition for Oxidation of silyl enol ether oxidation to the corresponding enones.
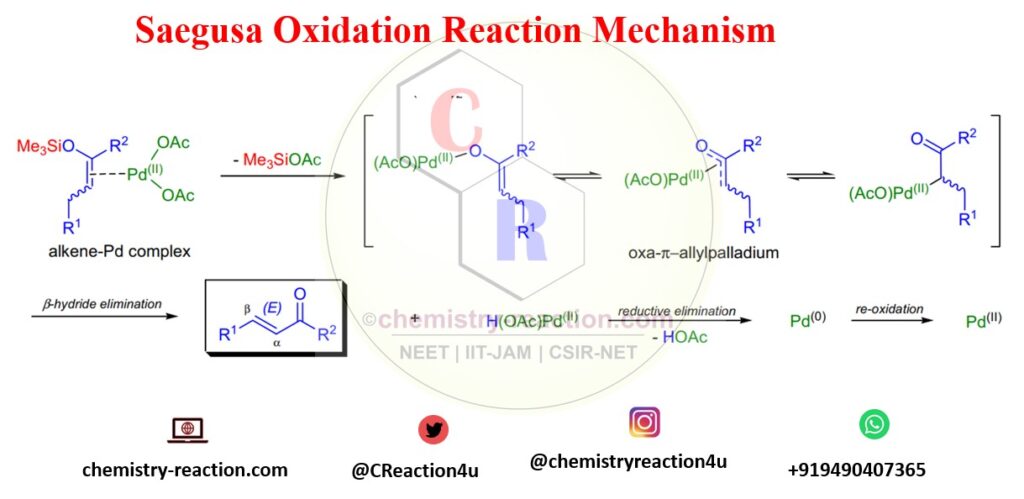
Saegusa Oxidation Mechanism:
Here this Oxidation Mechanism are shown When substoichiometric/stoichiometric amounts of Pd(OAc)2 is used: The mechanism involves the coordination of palladium to the enol olefin by leaving Me3SiOAc and form an oxoallyl-palladium complex. palladium hydride enone complex is unstable in nature and further undergoes β-hydride elimination leading product.
Saegusa Oxidation Applications:
- The first total synthesis of (–)-preussomerin and G (–)-gambierol the marine polycyclic ether toxin
- Total synthesis of (±)-8,14-cedranoxide by using Larock modified oxidation conditions
Related Reaction:
| Baeyer-Villiger Oxidation | Corey-Kim Oxidation |
| Criegee Oxidation | Dakin Oxidation |
| Davis’ Oxaziridine Oxidations | Dess-Martin Oxidation |
| Fleming-Tamao Oxidation | Jones Oxidation/ Oxidation of Alcohols by Chromium Reagents |
| Kornblum Oxidation | Ley Oxidation |
| Oppenauer Oxidation | Pfitzner-Moffatt Oxidation |
| Pinnick Oxidation | Swern Oxidation |
| Riley Selenium Dioxide Oxidation | Rubottom Oxidation |
| Wacker Oxidation | Wacker Oxidation |
References :
- J. Am. Chem. Soc. 1975, 97, 3, 649–651
- https://onlinelibrary.wiley.com/doi/10.1002/9780470638859.conrr556
- https://www.sciencedirect.com/topics/chemistry/saegusa-oxidation
- https://en.wikipedia.org/wiki/Saegusa%E2%80%93Ito_oxidation
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.
