Krapcho reported that upon heating geminal dicarbethoxy compounds with sodium cyanide in dimethyl sulfoxide, the corresponding ethyl esters were obtained in high yield.
Table of Page Contents
What is Krapcho Decarboxylation explain?
The Krapcho dealkoxycarbonylation is dealkoxycarbonylation of β-keto esters, α-cyano esters, malonate esters, and α-alkyl- or arylsulfonyl esters to the corresponding ketones, nitriles, esters, and alkyl- or arylsulfones is known as the Krapcho dealkoxycarbonylation and (also Krapcho reaction or Krapcho decarboxylation).

EWG = CO2-alkyl, CO2-aryl, CN, CO-alkyl, SO2-alkyl, SO2-aryl; R1-2 = H, alkyl, aryl; R3 = Me, Et; MX = NaCN, KCN, LiCl, NaCl, NaBr, NaI, LiI·H2O, Na2CO3·H2O, Na3PO4·12H2O, Me4NOAc ; solvent: DMSO, DMF, DMA, HMPT
Krapcho Decarboxylation Mechanism:
The Krapcho dealkoxycarbonylation mechanism is dependent on the the type of anion used structure of the substrate ester and . In the case of α,α-disubstituted diesters (especially the methyl esters), the anion from the salt (cyanide ion in the scheme) attacks the alkyl group of the ester in an SN2 fashion and the decarboxylation results in the formation of a carbanionic intermediate that is quenched by the water. In the case of α monosubstituted diesters the cyanide attacks the carbonyl group to form a tetrahedral intermediate, which breaks down to give the same carbanionic intermediate and a cyanoformate, which is hydrolyzed to give carbon dioxide and an alcohol.
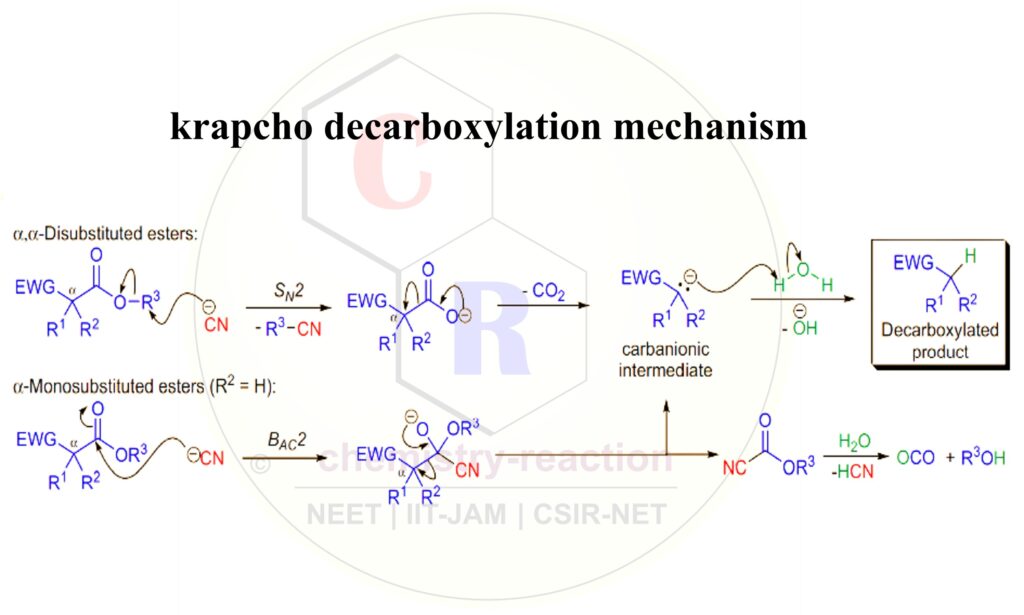
Related reactions:
References:
- Flynn, Daniel L.; Becker, Daniel P.; Nosal, Roger; Zabrowski, Daniel L. (1992-11-24). “Use of atom-transfer radical cyclizations as an efficient entry into a new “serotonergic” azanoradamantane”. Tetrahedron Letters. The International Journal for the Rapid Publication of Preliminary. 33 (48): 7283–7286. doi:10.1016/S0040-4039(00)60166-1
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

