What is Biginelli reaction?
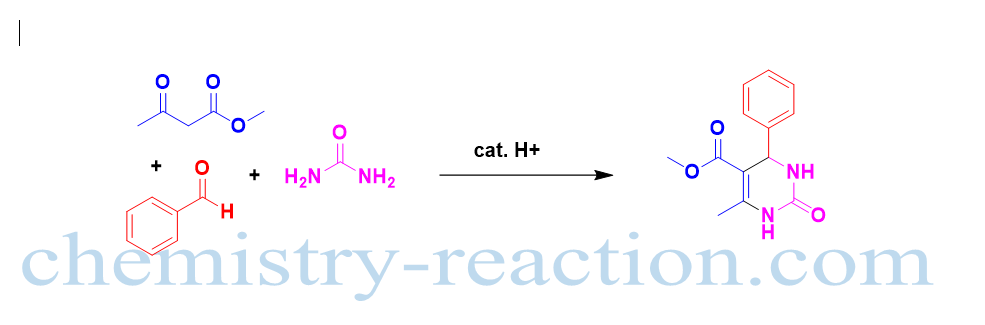
The acid catalysed three-component one-pot synthesis of highly functionalized 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) from biginelli reaction reactants heteroaromatic, aromatic and aliphatic aldehyde, urea, and ethyl acetoacetate.
The importance of biginelli reaction and application of biginelli reaction is to synthesize various drug molecules and the natural product synthesis. Biginelli reaction is widely utilized for the synthesis of biologically active compounds. Usually, transformation is carried out with a catalytic amount of Brönsted acid (LiBr, InBr3, BF3·OEt2, FeCl3, Yb(OTf)3, and Bi(OTf)3 ) and Lewis acids (HCl, H2SO4, and TsOH) alcoholic solvents. Shutalev modification and Atwal modification are the extended versions of reaction with better yield. Yeast catalyzed production 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) reported by Atul Kumar with enzymatic preparation.
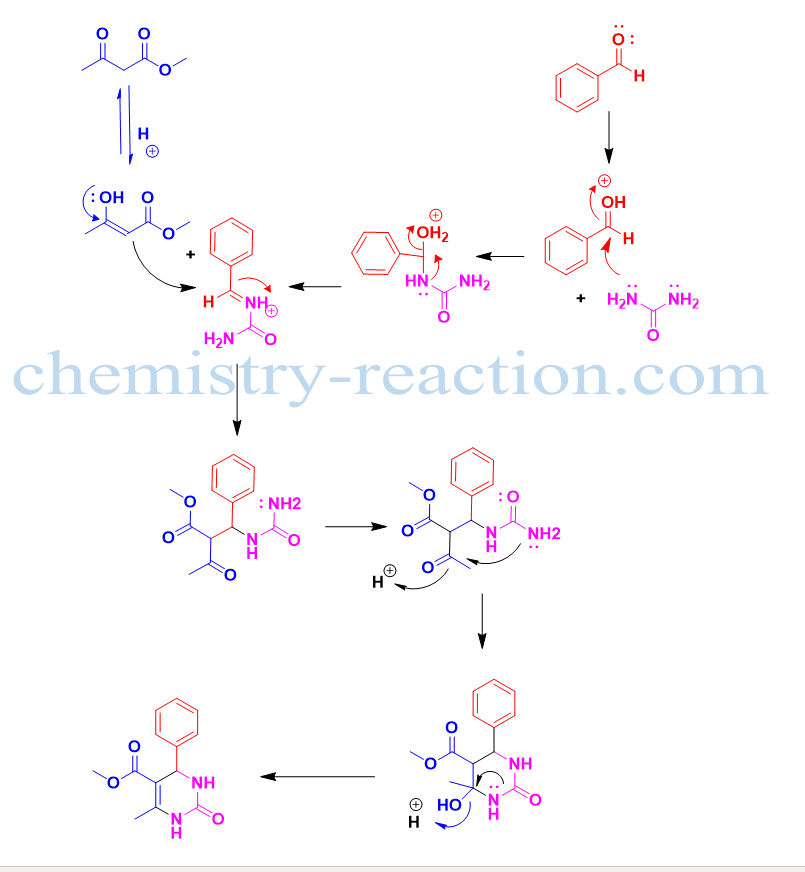
Biginelli reaction mechanism –
Biginelli reaction is an example of an acid-catalyzed three-component reaction. The first step in the mechanism of the Biginelli reaction is the acid protonates the aldehyde. Nucleophilic NH2 from urea will attack o electrophilic aldehyde by leaving water molecules from the N-acyliminium ion intermediate will generate. Then enol of β-keto ester will come into the picture and attack on N-acyliminium and generate chain ureide intermediate which will further convert into product i.e 3,4-dihydropyrimidin-2(1H)-ones.
My name is Pradip Sanjay W. I’m an organic chemist originally from Maharashtra, India. I have qualified UGC NET-JRF, GATE in chemical sciences and MH-SET exam for assistant professor. I’m currently pursuing my Ph.D. in organic chemistry at the Indian Institute of Technology Hyderabad, India.

